Kenneth Nwosu and Christopher M. Bono
INTRODUCTION
Spontaneous spondylodiscitis, or a primary infection of the nucleus pulposus with secondary involvement of the cartilaginous endplate and vertebral bone, was initially described by Ghormley et al. in 1940.1 However, it was not until the 1950’s when it was first documented as a complication of disc surgery.2
Since these early reports, spondylodiscitis has been diagnosed more frequency directly correlating with an increased rate of invasive spinal procedures being performed.3 This increase is also reflective of an expanding elderly and immunocompromised population as well as a heightened ability to diagnosis spinal infections through diagnostic advancements that have become available over time.
Although postoperative spondylodiscitis (POSD) is fortunately rare (in contrast to postoperative wound infection), its presentation is typically nonspecific, which can lead to delays in diagnosis and treatment, and consequently a suboptimal outcome. Of note, postoperative spinal infections have been reported to be associated with a twofold increase in mortality, fivefold increase in hospital readmission, fourfold increase in financial burden, and 60% greater chance of intensive care unit admission.4-6 The importance of appreciating subtle clinical findings and having a high clinical suspicion for POSD cannot be overstated. It is the goal of this chapter to review and discuss the epidemiology, pathogenesis, clinical features, and management of this potentially devastating condition.
INCIDENCE
The incidence of spontaneous discitis ranges from 0.4 to 2.4 per 100,000 each year.7,8 The age distribution is bimodal (young children and elderly) with males 1.5 to 3 times more often affected than females.9,10 Risk factors include age, diabetes, immunosuppression, intravenous drug use, alcoholism, hepatic cirrhosis, malignancy and renal failure.11-13 The incidence of POSD ranges from 0.2% to 3.6%,14-18 though it accounts for 30% of all cases of pyogenic discitis, with staphylococci and streptococci being the most common offending organisms.
Work-up of disc degeneration and its ensuing cascade of events are the most common reasons for diagnostic assessment and treatment of the lumbar spine. Unfortunately, these very modalities are often invasive and contribute to the incidence of iatrogenic infection. POSD is a known risk factor with a number of procedures including discography, myelography, chemonucleolysis, epidural injections, lumbar punctures, and virtually any lumbar surgery. On a relative scale, POSD is rare when the entire disc is removed anteriorly and replaced with bone graft, such as performed with anterior lumbar interbody fusion (ALIF)19 and when the disc is not entered at all (e.g. laminectomy alone).20
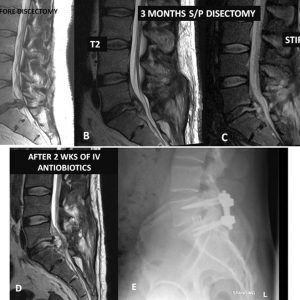
Discography, a controversial, provocative diagnostic technique used by some in the work-up of low back pain, warrants further discussion. The risk of discitis following discography performed under sterile conditions by an experienced discographer is approximately 1% when a single needle is used and up to 5% with a two-needle technique.21 Other investigators indicate that the incidence is underreported even in ideal circumstances, occurring in as many as 1 of every 30 patients.22,23 POSD also has the potential to evolve into an epidural abscess (Fig. 5-1) that may have significant neurological consequences.
While many feel minimally invasive surgery decreases the incidence postoperative infections, POSD has been reported in both minimally and maximally invasive procedures. Abdelrahman et al. reported a 0.46% incidence of POSD following vertebral cement augmentation,24 finding that immunosuppressed patients were at higher risk. Microdiscectomy with appropriate pre-incision prophylactic antibiotics has a reported 0.7% incidence of postoperative discitis, which reportedly doubles with use of a microscope.25 However, another study contradicts this, reporting a 0.4% incidence of POSD following discectomy with a microscope and a 2.8% incidence when a traditional open technique was used.26 Ultimately, there is no conclusive evidence that the use of a microscope influences the incidence of POSD.27,28
The incidence of post-operative wound infection following spinal fusion has been consistently reported to be higher when instrumentation is utilized. Prior to the use of routine antibiotic prophylaxis, Moe et al. reported a 2% infection rate after uninstrumented spinal fusion, 7% with the use of Harrington rods, and a 20% occurrence in adult posterior instrumented fusion for scoliosis.29 With antibiotics, the infection rate with use of Harrington rods was halved; from 7% to 3.6%.30 As the use of spinal instrumentation increases, infection rates as high as 12% have been reported.31,32
Studies specifying the incidence of POSD after fusion surgery are scant and mainly limited to case reports. Carmouche et al. reported a case of POSD 12 weeks following PLIF that was initially treated with intravenous antibiotics.33 The following week the patient was found to have an epidural abscess after which surgical debridement and abscess drainage was performed. The interbody device was left in place. However, in the ensuing 3 weeks, the patient worsened clinically and radiographically after which his interbody device and pedicle screws were removed, 4 months after his index surgery. His symptoms resolved shortly thereafter.
Other studies differentiate between superficial and deep post-operative infections without specifically assessing the incidence of POSD. Fritzell et al. made this distinction without further subcategorizing deep infections as POSD.34 Their multicenter randomized report included 211 patients that were followed for two years. They attempted to compare complication rates between patients that underwent uninstrumented posterolateral fusion, instrumented posterolateral fusion and instrumented posterolateral fusion with interbody fusion (so-called 360 degree fusion). They reported seven infections, of which five were deep. Deep infections occurred in four patients that underwent instrumented fusions and one who underwent 360 degree fusion. There were no deep infections in patients who underwent uninstrumented fusions.
One cohort study was able to further distinguish POSD from postoperative posterior infection following posterior instrumented fusion. Hsieh et al. reported on 6,120 that underwent posterolateral instrumented fusions for spondylosis or trauma.35 They reported a 2.2% posterior infection rate and 0.002% POSD. Nine of the 11 patients with POSD were also found to have deep posterior infections. Interestingly, they reported a symptom free interval of 20 weeks on average (2 – 24 wks) for those that were subsequently diagnosed with POSD. This delay may be attributable to the period of time required for a deep infection to contiguously spread anteriorly to an adjacent osteodiscal complex.
Ultimately, the true incidence of POSD following uninstrumented or instrumented fusion is unknown due to a paucity of studies differentiating between superficial infection, deep infection, and anterior osteodisicitis following these operations. However, given the intimate communication and potential for contiguous seeding between infectious pathogens deep to the facia and its adjacent osteodiscal complex, one may postulate the rate of POSD is slightly lower but closely correlates with deep infection rates following fusion surgery. This correlation is likely more significant in the subgroup of deep postoperative infections that are not diagnosed and treated expeditiously. This is further supported by the symptom free interval difference from index surgery to POSD between patients that have undergone disc surgery and those that received posterior instrumented fusion, and the high rate (82%) of deep infection among patients who were found to have POSD following posterior instrumented fusion.35
PATHOGENESIS
POSD are usually pyogenic; however, tuberculous and fungal spondylodiscites have been described. There are many potential sources of intraoperative contamination. Weiner et al. showed the use of a headlamp, loupes or the operative microscope was associated with bacterial shedding.36 Implant handling can also be a source. Bible et al. demonstrated that uncovered implant trays have a 16.7% contamination rate (as assessed by swab and culture) while covered trays were contaminated in only 2.0% of cases.37 Other sources of contamination include the C-arm/fluoroscope38 as well as surgeons and assistants. The use of pre-incision, intravenous antibiotic prophylaxis has the most significant effect in decreasing postoperative infection rates.39,40 This demonstrates that despite adequate preparation and draping, skin flora remain an important potential contaminant.
Direct inoculation is the most likely mode of pathogen seeding in adult POSD given an avascular disc space. Conversely, the pediatric population is more susceptible to true spontaneous hematogoenous discitis given their relatively vascular intervertebral discs.41 Following direct inoculation from a procedure that directly involves the intervertebral disc, development of an “early” (<20 weeks) POSD is theorized to occur relatively soon after index surgery. In contrast, a “delayed” (>20 weeks) POSD, often after procedures that do not come in direct contact with the disc, is thought to be a result of contiguous or hematogenous seeding of an at-risk surgical site with significant dead space and poor vasculature.35 In delayed POSD, the vertebral body and intervertebral disc may be affected simultaneously following contiguous seeding. With hematogenous seeding, the vertebral body is often affected first, with subsequent contiguous spread to the intervertebral disc. This distinction is valuable towards investigating and eradicating potential distant sources of infection when a delayed POSD is suspected. It may also guide treatment in that implants may be retained during treatment of early POSD while implant removal should be strongly considered during treatment of delayed POSD, especially when a solid fusion is present.35
Postoperative Pyogenic Spondylodiscitis
The most common cause of pyogenic POSD has consistently been shown to be Staphylococcus aureaus (60%) followed by other Staphylococcus species and anaerobic organisms.42 Less common organisms include streptococcus species, Escherichia coli and Pseudomonas aeruginosa. Hsieh et al. report that organisms cultured from 11 POSD cases included methicillin-resistant coagulase negative staphylococci in two cases, methicillin-sensitive coagulase negative staphylococci in two cases, methicillin-resistant Staphylococcus aureus in two cases, methicillin-sensitive Staphylococcus aureus in one case and Serratia marcescens in two cases. Katonis et al. reviewed 18 patients who had POSD after posterior lumbar spine surgery including discectomy, laminectomy and instrumented fusions.43 They found that three patients developed an epidural abscess. Of the three, tissue culture grew methicillin-resistant Staphylococcus aureus in one patient and Serratia marcescens in two. In all the patients, these organisms were also found on blood cultures and correlated with tissue culture growth. Propionibacterium acnes and diptheroids, often skin flora, are sometimes responsible for delayed or chronic postoperative deep infections.44
From a preventative standpoint, vancomycin powder has been extensively studied and shown to decrease the incidence of surgical site infection, including deep infections and spondylodiscitis.45-47 Lee et al. reported the deep infection rate with methicillin-sensitive staphylococcus aureus (MSSA) decreased from 2.7% to 0.7%, and methicillin-resistant staphylococcus aureus (MRSA) infection decreased from 1.6% to 0.4% in the non-vancomycin powder group compared to the vancomycin powder group, respectively.45 Khan et al., in a subgroup analysis, found that patients in whom spinal implants were used had a reduced risk of surgical site infection with vancomycin powder (P=0.023), compared with those who had non-instrumented spinal operations (p=0.226).46 However, upcoming studies suggest that although gram positive surgical site infection rates are decreased with use of vancomycin powder, the rate of more troublesome gram negative infections may be on the rise.48
Postoperative Tuberculosis Spondylodiscitis
Postoperative tuberculous spondylodiscitis is extremely rare. It is typically contracted from a non-detectable source hematogenously, most often an indolent pulmonary focus.49 Diagnosis is often difficult with nonspecific clinical signs and symptoms and a protracted investigatory period prior to diagnosis. In two separate case reports, postoperative tuberculosis spondylodiscitis diagnosis was not made until three months after index surgery.50,51 In the first, the patient underwent two operations prior to diagnosis. In the second, the patient underwent three operations prior to diagnosis. In both cases, each patient received several months of antibiotics without resolution of symptoms prior to diagnosis. In another report, diagnosis was not made until eight years after index L4-L5 discectomy.51
Postoperative Fungal Spondylodiscitis
Postoperative fungal spondylodiscitis is also very rare. Its most probable source is direct invasion of fungus into the disc space during the primary procedure. There are two reports encompassing 14 cases of postoperative fungal spondylodiscitis.52,53 Nine of the cases were due to mold, five were caused by Candida species. Median age was 49 years old. Most (31%) of the cases followed discectomy, 21% after laminectomy and discectomy and 14% after laminectomy alone. Aspergillus was the culprit 43% of the time and Candida 36%. Back pain was the most common symptom and often began approximately six weeks after index procedure.
Postoperative fungal spondylodiscitis often has a good prognosis after surgical debridement and antifungal therapy; however, reaching a diagnosis may be challenging due to a lack of suspicion. Hence, it is important to consider a fungal etiology in all cases suspicious of POSD, especially after culture results from a biopsy site is reportedly negative for bacteria while symptoms persist. Garcia et al. highlighted the importance of ventilation filters in the operating room that are able to remove airborne particles over five micrometer, of which most mold particles are greater.54
CLINICAL PRESENTATION
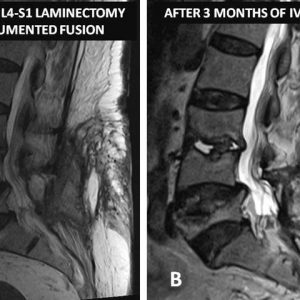
Patients with POSD most often presents with pain after a symptom-free interval.55 As a result, the diagnosis may be difficult and delayed because clinicians may mistake spondylodiscitis pain from post-operative surgical pain (Fig. 5-2). In their series of 17 patients with postoperative spondylodiscitis after lumbar spine surgery, Bavinzki et al. reported all patients had localized pain characterized as sharp and radiating exacerbated by movement and associated with muscle spasms.56 Thirteen patients had a positive straight leg raise and six had fevers. However, the clinical presentation may be more indolent. For example, Harris et al. reported on a case of Propionibacterium acnes where the patient had mild pain at the surgical site that began approximately three months after surgery.57 This patient was afebrile and despite appropriate antibiotic and initial surgical treatment, the disease progressed causing permanent lower extremity weakness requiring the patient to use a walker for ambulation.
One study reported the diagnosis of POSD versus spontaneous spondylodiscitis was made on average 16 weeks after the onset of symptoms versus 3.4 weeks, respectively, highlighting the potential difference in presentation between the two entities.20 Dufour et al. reported on 7 and 16 patients with POSD and spontaneous spondylodiscitis, respectively, out of 2400 spine operations.20 POSD patients tended to be younger with less frequent underlying disease. Blood cultures were positive in POSD versus spontaneous spondylodiscitis 14% of the time versus 81%, respectively. Coagulase-negative staphylococci were more commonly isolated in POSD. Vertebral edema was more frequent and posterior in POSD
Designating acuity of POSD is important towards providing adequate treatment. POSD occurring within four weeks of clinical symptoms is designated as acute. Those occurring for greater than four weeks after clinical symptoms began are designated as chronic.
Acute POSD
The most common presentation is severe pain one to four weeks after surgery and is by convention deemed acute. Pain is usually out of proportion to the clinical findings and sometimes mimics a recurrent disc herniation. Constitutional symptoms are rare after discectomies but are more commonly experienced following complex spine surgeries including those in which instrumentation is used. Rawlings et al. reported 27% of patients with postoperative spondylodiscitis had radiculopathy, 13% had constitutional symptoms and less than 10% had incisional signs of infection.58 Bassewitz et al. reported 16% progress to having an epidural abscess. Patients with epidural abscesses typically progress symptomatically from back pain to radiculopathy after which weakness and paralysis may ensue within seven to ten days.59 Less often, along with neurological signs, patients may also suffer from meningeal irritation and neck rigidity.
Acute POSD is less common in the cervical compared to the lumbar spine.58,60 Cervical spine POSD primarily presents with neck pain, restricted range of motion and sometimes torticollis.61 Those that progress to have a retropharyngeal abscess may complain of throat pain with difficulty swallowing and can subsequently progress to respiratory distress.
Chronic POSD
Progression to chronic POSD is often due to a lack of diagnosis in the early stages. Symptoms typically include back or neck pain, muscle spasms, difficulty walking and night pain. These patients are also more likely to complain of malaise and have low-grade fevers. Chronic cervical POSD often presents with persistent central nuchal pain and sometimes myeloradiculopathy.62 Esophageal fistula is a known complication of POSD after anterior cervical instrumented fusion. It is important to also recognize that persistent pain, redness and swelling, with or without discharge may be due to a retained sponge/foreign body in the wound. Chronic POSD is more likely to be associated with elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and white blood cell (WBC) counts.
IMAGING
Magnetic resonance imaging (MRI) is the diagnostic modality of choice in detecting POSD. It can show changes well before plain films or even computerized tomography (CT). Early MRI findings can include increased signal intensity within the disc space on T2 of STIR-weighted images and peri-discal edema (see Figs. 5-1 and 5-2). MRI can also clearly delineate associated epidural abscesses. Gadolinium-enhanced MRI’s are popular in the postoperative setting, primarily because of the distinction between recurrent disc herniations that do not enhance and scar that does enhance. Enhancement can also be seen in areas of infection with rim enhancement of fluid collections particularly noteworthy. In the authors’ experience, however, Gadolinium-enhanced MRI’s are not routinely needed in the postoperative setting and in evaluating POSD.
CT scans delineate bony changes best, which is usually a later finding with POSD. CT can be useful in examining the disc space for autofusion, an ideal result of successful nonoperative management of discitis. Plain films have less of a role in early detection but more so for serial follow-up.
MEDICAL MANAGEMENT
The majority of POSD cases can be treated non-surgically. The goals of medical treatment are to control pain, alleviate infection, prevent relapse and restore function without subjecting the patient to the morbidity associated with revision surgery. This is often accomplished with six weeks of intravenous antibiotics and is associated with acceptable long term results.55,63Patients are often initially treated empirically to cover S. Aureus, the most common culprit. A more ideal approach, however, involves identifying the causative organism and its antibiotic susceptibilities either via blood or tissue microbiology testing towards facilitating an appropriate pathogen-specific antimicrobial regimen. Following completion of intravenous antibiotic treatment, some infectious disease specialists recommend maintaining patients indefinitely on oral suppressive organism-specific antibiotics especially if instrumentation was used and is retained.
A response to treatment can and should be monitored prior to cessation of antibiotics. This is accomplished via serial CRPs and ESRs, as well as imaging. Serum CRP level is closely related with clinical response to therapy and is, therefore, the preferred marker for the course of infection.64 Criteria for discontinuation of antimicrobial therapy include resolution of clinical symptoms as well as normalization of CRP.65 It has been proposed that a weekly 50% decrease in CRP represents treatment response; a lack of improvement in symptoms as well as a persistently elevated CRP above 30 mg/l are predictors of treatment failure.66
Although monitoring treatment with MRI in unoperated patients is often straightforward, it is typically more challenging following surgery. The operated level often shows post-surgical inflammatory changes that may include edema of the vertebral marrow adjacent to the disc and contrast enhancement of the disc and its adjacent endplates. A decrease in enhancing soft tissue mass at the suspected level in the paravertebral muscle and in the epidural space is often indicative of a positive response to treatment. Of note, bone marrow edema and increased disc T2 signal intensity can still be seen after six months of clinical and biochemical resolution.67 However, a transition from low to high T1 signal intensity with treatment correlates well with clinical recovery.68 Ongoing loss of vertebral disc height does not necessarily indicate treatment failure and can accompany clinical improvement.69 However continued bony destruction on follow up should prompt review of the diagnosis and treatment.
SURGICAL MANAGEMENT
Surgical indications for POSD include clinical progression of infection and worsening MRI findings despite appropriate antibiotic treatment, progression of the infection into the spinal canal causing severe pain or neurological symptoms, progressive vertebral destruction causing deformity and instability, and sepsis. When indicated, single stage anterior and posterior debridement, reconstruction and fusion is effective.70
POSD associated with paravertebral fluid accumulation rarely responds to antibiotic therapy alone. Surgical debridement with removal of all necrotic tissues is usually the first line of treatment in this scenario. The presence of posterior instrumentation, and the question of whether to remove or retain them, further complicates the management plan. In addition to meticulous debridement and copious irrigation, instrumentation should be maintained when possible to avoid creating instability and loss of deformity correction. Loose instrumentation should be removed and replaced if needed. Ultimately, both bone screws and interbody devices may be left in place with early postoperative infections.44,71,72 Delayed wound closure via vacuum-dressing and repeat irrigation and debridement are often employed. In cases of late infection with a solid fusion mass, screws and hardware may be removed to facilitate infection clearance.73 Tight blood sugar control and nutritional optimization should be rigorously sought in all patients. Intravenous antibiotics is usually continued for at least six weeks postoperatively after which select patients should be maintained on tailored oral suppressive antibiotics depending on the causative pathogen, patients health status and presence of spinal instrumentation.
SUMMARY
Although the incidence of POSD is rare, its associated morbidity and healthcare cost are disproportionately high. Over time, as we have become more knowledgeable regarding factors that contribute to POSD, preventative measures and protocols have been proposed and adopted. Yet, paradoxically, the incidence of POSD has remained unchanged and, according to some, increased. This discrepancy is thought to be a result of the aging population, increased utility of spinal instrumentation and increased rate of complex spine surgeries being performed. The true challenge of POSD lies in its timely diagnosis, especially after reports have consistently shown morbidity is significantly improved with early treatment. Hence, there is significant value for spine care providers to understand and mitigate risk factors that may contribute to POSD. When present, POSD will likely be pyogenic. However, the spine care provider must also consider other sources like tuberculosis and fungal POSD. Medical treatment with intravenous antibiotics is typically the first line of treatment and is often effective, but surgery is required in select cases. If surgery is deemed necessary, spinal implants may be retained in early infections but should be removed if a solid fusion mass is demonstrated.
REFERENCES
- Ghormley RK, Bickel WH, Dickson DD. A study of acute infectious lesions of the intervertebral disks. Southern Med J. 1940;33:347-353.
- Turnbull F. Postoperative inflammatory disease of lumbar discs. J Neurosurg. 1953;10(5):469-473.
- Vergne P, Trèves R. [Infectious spondylodiscitis. Etiology, diagnosis, progression and treatment]. Rev Prat. 1998;48(18):2065-2071.
- Calderone RR, Garland DE, Capen DA, Oster H. Cost of medical care for postoperative spinal infections. Orthop Clin North Am. 1996;27(1):171-182.
- Watters WC 3rd, Baisden J, Bono CM, et al. Antibiotic prophylaxis in spine surgery: an evidence-based clinical guideline for the use of prophylactic antibiotics in spine surgery. Spine J. 1009;9(2):142-146.
- Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol. 1996;17(8):552-557.
- Lam KS, Webb JK. Discitis. Hosp Med. 2004;65(5):280-286.
- Hopkinson N, Stevenson J, Benjamin S. A case ascertainment study of septic discitis: clinical, microbiological and radiological features. QJM. 2001;94(9):465-470.
- Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976). 2000;25(13):1668-1679.
- Colmenero JD, Jiménez-Mejías ME, Sánchez-Lora FJ, et al. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997;56(12):709-715.
- Khan IA, Vaccaro AR, Zlotolow DA. Management of vertebral diskitis and osteomyelitis. Orthopedics. 1999;22(8):758-765.
- Govender S. Spinal infections. J Bone Joint Surg Br. 2005;87(11);1454-1458.
- Friedman JA, Maher CO, Quast LM, McClelland RL, Ebersold MJ. Spontaneous disc space infections in adults. Surg Neurol. 2002;57(2):81-86.
- Lindholm TS, Pylkkänen P. Discitis following removal of intervertebral disc. Spine (Phila Pa 1976). 1982;7(6):618-622.
- El-Gindi S, Aref S, Salama M, Andrew J. Infection of intervertebral discs after operation. J Bone Joint Surg Br. 1976;58(1):114-116.
- Silber JS, Anderson DG, Vaccaro AR, Anderson PA, McCormick P; NASS. Management of postprocedural discitis. Spine J. 2002;2(4):279-287.
- Deyo RA, Cherkin DC, Loeser JD, Bigos SJ, Ciol MA. Morbidity and mortality in association with operations on the lumbar spine. The influence of age, diagnosis, and procedure. J Bone Joint Surg Am. 1992;74(4):536-543.
- Jiménez-Mejías ME, de Dios Colmenero J, Sánchez-Lora FJ, et al. Postoperative spondylodiskitis: etiology, clinical findings, prognosis, and comparison with nonoperative pyogenic spondylodiskitis. Clin Infect Dis. 1999;29(2):339-345.
- Cloward RB. Metastatic disc infection and osteomyelitis of the cervical spine. Surgical treatment. Spine (Phila Pa 1976). 1978;3(3):194-201.
- Dufour V, Feydy A, Rillardon L, et al. Comparative study of postoperative and spontaneous pyogenic spondylodiscitis. Semin Arthritis Rheum. 2005;34(5):766-771.
- Camillo, F. Arthrodesis of the spine. In Campbell W, Canale ST, Beaty J, eds. Campbell’s Operative Orthopaedics, 4th ed. Philadelphia, PA: Mosby Elsevier; 2008).
- Connor PM, Darden BV 2nd. Cervical discography complications and clinical efficacy. Spine (Phila Pa 1976). 1993;18(14):2035-2038.
- Fraser RD, Osti OL, Vernon-Roberts B. Discitis after discography. J Bone Joint Surg Br. 1987;69(1):26-35.
- Abdelrahman H, Siam AE, Shawky A, Ezzati A, Boehm H. Infection after vertebroplasty or kyphoplasty. A series of nine cases and review of literature. Spine J. 2013;13(12):1809-1817.
- Sasso RC, Garrido BJ. Postoperative spinal wound infections. J Am. Acad Orthop Surg. 2008;16(6):330-337.
- Dauch WA. Infection of the intervertebral space following conventional and microsurgical operation on the herniated lumbar intervertebral disc. A controlled clinical trial. Acta Neurochir (Wien). 1986;82(1-2):43-49.
- Tronnier V, Schneider R, Kunz U, Albert F, Oldenkott P. Postoperative spondylodiscitis: results of a prospective study about the aetiology of spondylodiscitis after operation for lumbar disc herniation. Acta Neurochir (Wien). 1992;117(3-4):149-152.
- Wilson DH, Harbaugh R. Microsurgical and standard removal of the protruded lumbar disc: a comparative study. Neurosurgery. 1981;8(4):422-427.
- Moe JH. Complications of scoliosis treatment. Clin Orthop Relat Res. 1967;53:21-30.
- Dobzyniak MA, Fischgrund JS, Hankins S, Herkowitz HN. Single versus multiple dose antibiotic prophylaxis in lumbar disc surgery. Spine (Phila Pa 1976). 2003;28(21):E453-455.
- Rechtine GR, Bono PL, Cahill D, Bolesta MJ, Chrin AM. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma. 2001;15(8):566-569.
- Sponseller PD, LaPorte DM, Hungerford MW, Eck K, Bridwell KH, Lenke LG. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine (Phila Pa 1976). 2000;25(19):2461-2466.
- Carmouche JJ, Molinari RW. Epidural abscess and discitis complicating instrumented posterior lumbar interbody fusion: a case report. Spine (Phila Pa 1976). 2004;29(23):E542-546.
- Fritzell P, Hägg O, Nordwall A; Swedish Lumbar Spine Study Group. Complications in lumbar fusion surgery for chronic low back pain: comparison of three surgical techniques used in a prospective randomized study. A report from the Swedish Lumbar Spine Study Group. Eur Spine J. 2003;12(2):178-189.
- Hsieh MK, Chen LH, Niu CC, Fu TS, Lai PL, Chen WJ. Postoperative anterior spondylodiscitis after posterior pedicle screw instrumentation. Spine J. 2011;11(1):24-29.
- Weiner BK, Kilgore WB. Bacterial shedding in common spine surgical procedures: headlamp/loupes and the operative microscope. Spine (Phila Pa 1976). 2007;32(8):918-920.
- Bible JE, O’Neill KR, Crosby CG, Schoenecker JG, McGirt MJ, Devin CJ. Implant contamination during spine surgery. Spine J. 2013;13)16):637-640.
- Biswas D, Bible JE, Whang PG, Simpson AK, Grauer JN. Sterility of C-arm fluoroscopy during spinal surgery. Spine (Phila Pa 1976). 2008;33(17):1913-1917.
- Rohde V, Meyer B, Schaller C, Hassler WE. Spondylodiscitis after lumbar discectomy. Incidence and a proposal for prophylaxis. Spine (Phila Pa 1976). 1998;23(5):615-620.
- Schulitz KP, Assheuer J. Discitis after procedures on the intervertebral disc. Spine (Phila Pa 1976). 1994;19(10):1172-1177.
- Skaf GS, Domloj NT, Fehlings MG, et al. Pyogenic spondylodiscitis: an overview. J Infect Public Health. 2010;3(1):5-16.
- Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976). 2005;30(12):1460-1465.
- Katonis P, Tzermiadianos M, Papagelopoulos P, Hadjipavlou A. Postoperative infections of the thoracic and lumbar spine: a review of 18 cases. Clin Orthop Relat Res. 2007;454:114-119.
- Weinstein MA, McCabe JP, Cammisa FP Jr. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13(5):422-426.
- Lee GI, Bak KH, Chun HJ, Choi KS. Effect of using local intrawound vancomycin powder in addition to intravenous antibiotics in posterior lumbar surgery: midterm result in a single-center study. Korean J Spine. 2016;13(2):47-52.
- Khan NR, Thompson CJ, DeCuypere M, et al. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine. 2014;21(6):974-983.
- Kang DG, Holekamp TF, Wagner SC, Lehman RA Jr. Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J. 2015;15(4):762-770.
- Ghobrial GM, Thakkar V, Andrews E, et al. Intraoperative vancomycin use in spinal surgery: single institution experience and microbial trends. Spine (Phila Pa 1976). 2014;39(7):550-555.
- Almeida A. Tuberculosis of the spine and spinal cord. Eur J Radiol. 2005;55(2):193-201.
- Jeon DW, Chang BS, Jeung UO, et al. A case of postoperative tuberculous spondylitis with a bizarre course. Clin Orthop Surg. 2009;1(1):58-62.
- Lotfinia I, Vahedi P. Late-onset post-diskectomy tuberculosis at the same operated lumbar level: case report and review of literature. Eur Spine J. 2010;19 Suppl 2:S226-232.
- Tack KJ, Rhame FS, Brown B, Thompson RC Jr. Aspergillus osteomyelitis. Report of four cases and review of the literature. Am J Med. 1982;73(2):295-300.
- Kim KW, Ha KY, Kim MS, Choi SM, Lee JS. Postoperative Trichosporon asahii spondylodiscitis after open lumbar discectomy: a case report. Spine (Phila Pa 1976). 2008;33(4);E116-120.
- Garcia-Vidal C, Cabellos C, Ayats J, Font F, Ferran E, Fernandez-Viladrich P. Fungal postoperative spondylodiscitis due to Scedosporium prolificans. Spine J. 2009;9(9):e1-7.
- Ozuna RM, Delamarter RB. Pyogenic vertebral osteomyelitis and postsurgical disc space infections. Orthop Clin North Am. 1996;27(1):87-94.
- Bavinzski G, Schoeggl A, Trattnig S, et al. Microsurgical management of postoperative disc space infection. Neurosurg Rev. 2003;26(2):102-107.
- Harris AE, Hennicke C, Byers K, Welch WC. Postoperative discitis due to Propionibacterium acnes: a case report and review of the literature. Surg Neurol. 2005;63(6):538-541; discussion 541.
- Rawlings CE 3rd, Wilkins RH, Gallis HA, Goldner JL, Francis R. Postoperative intervertebral disc space infection. Neurosurgery. 1983;13(4):371-376.
- Bassewitz H, Herkowitz H. Lumbar stenosis with spondylolisthesis: current concepts of surgical treatment. Clin Orthop Relat Res. 2001;(384):54-60.
- Zeidman SM, Ducker TB, Raycroft J. Trends and complications in cervical spine surgery: 1989-1993. J Spinal Disord. 1997;10(6):523-526.
- Calderone RR, Larsen JM. Overview and classification of spinal infections. Orthop Clin North Am. 1996;27(1):1-8.
- Ghanayem AJ, Zdeblick TA. Cervical spine infections. Orthop Clin North Am. 1996;27(1):53-67.
- Visuri T, Pihlajamäki H, Eskelin M. Long-term vertebral changes attributable to postoperative lumbar discitis: a retrospective study of six cases. Clin Orthop Relat Res. 2005;(433):97-105.
- Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J. 2006;6(3):311-315.
- McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
- Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Osmon DR. Do follow-up imaging examinations provide useful prognostic information in patients with spine infection? Clin Infect Dis. 2006;43(2):172-179.
- Van Goethem JW, Parizel PM, Jinkins JR. Review article: MRI of the postoperative lumbar spine. Neuroradiology. 2002;44(9):723-739.
- Leone A, Dell’Atti C, Magarelli N, et al. Imaging of spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16 Suppl 2:8-19.
- Nickerson EK, Sinha R. Vertebral osteomyelitis in adults: an update. Br Med Bull. 2016;117(1):121-138.
- Przybylski GJ, Sharan AD. Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg. 94, 2001;94(1 Suppl):1-7.
- Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2011;93(17):1627-1633.
- Sierra-Hoffman M, Jinadatha C, Carpenter JL, Rahm M. Postoperative instrumented spine infections: a retrospective review. South Med J. 2010;103(1):25-30.
- Viola RW, King HA, Adler SM, Wilson CB. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine (Phila Pa 1976). 1997;22(20):2444-2450; discussion 2450-2451.