Jose Antonio Umali and Ashish Diwan
INTRODUCTION
De Novo or spontaneous or primary pyogenic spondylodiscitis is an infection affecting both the vertebral body and intervertebral disc of the spine due to bacterial pathogens. Spontaneous pyogenic spondylodiscitis is caused by hematogenous spread secondary to bacteremia from integumentary, respiratory, oral or gastrointestinal infections.1,2 The origin of the infection can be categorized according to anatomic location, mode of transmission and infecting organism.
De Novo Spondylodiscitis can be spread either hematogenously or via contiguous spread from adjacent tissues. Spontaneous pyogenic spondylodiscitis is most commonly caused by hematogenous transmission of septic emboli to the vertebral body and intervertebral disc via arterial and venous circulations, particularly the Batson’s plexus. Batson’s plexus is the valveless network of veins within the vertebra that are connected with other venous networks within the body. The Batson’s plexus and its interconnections serve as potential routes for tumor emboli, air embolism and infections.3
Pathogens that lead to spontaneous pyogenic spondylodiscitis initially invade the end-arterial arcades present in the metaphyseal bone of the vertebral body adjacent to the disc. Septic emboli lodged within the metaphyseal region cause bone infarcts, leading to osteomyelitis. The infection then spreads and affects the adjacent disc via direct extension and rupture of the infective focus from the vertebral endplates.1,2
ETIOLOGY & MICROBIOLOGY
Common causative organisms of spontaneous pyogenic spondylodiscitis include Staphylococcus aureus, enterococci, streptococcus species and gram negative bacilli. Opportunistic pathogens rarely cause spondylodiscitis. Opportunistic pathogens include Mycobacterium tuberculosis, fungal infections and parasitic infestations. Immunocompromised patients are often affected by the opportunistic organisms.2
Staphylococcus aureus is the most commonly isolated organism in patients diagnosed with spontaneous pyogenic spondylodiscitis.4 It is estimated that about 50% of cases are caused by Staphylococcus aureus pathogen. Some reviews have stated that it is the causative organism in patients with de novo pyogenic spondylodiscitis ranging from 20 to 84%.4,5 Methicillin-sensitive Staphylococcus aureus was found to be more common compared to Methicillin-resistant Staphylococcus aureus (MRSA).6
Gram negative rods are found in 7 to 33% of cases. This group of organisms include Escerichia coli, Proteus, Klebsiella, Enterobacter spp and Pseudomonas aeruginosa.4,5 Pseudomonas aeruginosa is commonly associated with intravenous drug abusers, yet Staphylococcus aureus is still the main pathogen isolated.7,8
Streptococci and enterococci are reported to cause 5 to 20% of cases of spondylodiscitis. Streptococci infection was said to be associated with infective endocarditis or with a dental port of entry (viridans streptococci).4,5,9,10 Streptococcus pneumoniae is a rare source of spondylodiscitis.11
Anaerobic infections lead to spondylodiscitis in 3 to 4% of cases. Propionibacterium acnes and Bacteroides fragilis are the more common anaerobes associated with spondylodiscitis. Bacteroides fragilis is more commonly associated with patients diagnosed with diabetes mellitus and patients with pelvic or intra-abdominal infections.4,5,12,13
EPIDEMIOLOGY
Pyogenic spondylodiscitis has a bimodal age distribution, affecting patients aged less than 20 years and patients ranging from 50 to 70 years of age. In developed countries, the number of patients diagnosed with pyogenic spondylodiscitis range from 4 to 24 million.4,5 Men are more likely to be affected when compared to women (3:1).14 Studies have shown that pyogenic spondylodiscitis has had an increasing incidence from 2008 to 2011. The incidence of pyogenic spondylodiscitis was highest in the elderly population (more than 70 years old). A hospital based study in the UK showed the local incidence of spontaneous spondylodiscitis from 2008 to 2011 was 3.67/100000 per year, thus showing a 150% increase compared to the incidence in 1995 to 1999 (1.47/100000 per year). The increase in incidence was attributed to improvement in diagnostic methods, as well as an increasing elderly population.6,15
CLINICAL PRESENTATION
Pyogenic spondylodiscitis presents in patients as back pain, fever, as well as other neurologic symptoms. Back pain appears to be the most consistent symptom in patients (90%) and is noted to worsen in the evenings. This can be exacerbated by movement and may radiate to various areas of the body.1,4,16 Paravertebral muscle tenderness, spasms and restricted range of motion of the spine are also associated with back pain.
Fever is commonly associated with back pain and is found to be present in almost 50% of cases.4,5,16,17 Studies comparing spontaneous pyogenic spondylodiscitis with other forms of spondylodiscitis have reported that fever more than 38°C may be present in 56.6% to 62.4% of patients. A study by Turunc et al. comparing pyogenic spondylodiscitis with brucellar and tubercular spondylodiscitis showed that fever was less common in pyogenic spondylodiscitis (56.6%) as opposed to brucellar spondylodiscitis (84.3%) and tuberculous spondylodiscitis (76.9%). Contrary to this, the study by Yoon et al. showed that fever was more commonly encountered in patients with pyogenic spondylodiscitis (62.4%) compared to patients with tuberculous spondylodiscitis (28.3%).18-20
Neurologic symptoms are present in almost a third of patients. This can range from mild dyesthesia, weakness to radiculopathy, severe paralysis, or bowel and/or bladder incontinence secondary to sphincter loss.4,17,21 Progression of radiculopathy to weakness and paralysis may point to the presence of an epidural abscess or kyphotic deformity.1,16 Abscesses in the lumbar spine may encroach on the ischiatic foramen and cause compression on lumbosacral nerve roots leading to cauda equina syndrome.4
Spondylodiscitis in children may present with non-specific signs and symptoms. Most patients have low grade fever and seldom present with significant neurologic symptoms or deficits (10%). This leads to a delay in diagnosis and treatment as late as 4 to 6 months. Spondylodiscitis should be considered and further investigated when a child presents with back pain, reduced mobility or ambulation, or with unexplained irritability.22
Spontaneous pyogenic spondylodiscitis usually spreads hematogenously secondary to infection from the skin, subcutaneous tissues, urinary tract and other systems of the body. Certain patient risk factors have also been identified and associated with spontaneous pyogenic spondylodiscitis. These include diabetes mellitus, intravenous drug abuse, catheter-associated infections, surgical interventions, infective endocarditis, urinary tract infections, chronic alcoholism and immunocompromised states (Table 3-1).18,23
| Pathogen | Associated Infection/Co-morbidities |
|---|---|
| S. aureus | Skin infection Acute endocarditis |
| Streptococcus | Subacute endocarditis |
| Pseudomonas | Intravenous drug abuse |
| Enterococci | Urinary tract infection |
Gram negative organisms
|
Alcoholism |
Anaerobes
|
Diabetes mellitus Pelvic or intra-abdominal infections |
Spondylodiscitis in Special Settings
There are certain special settings that preclude patients to developing spondylodiscitis. Exposure to healthcare is recognized to be one such setting. Lillie et al. identified certain patients that developed healthcare acquired discitis. These patients were noted to have risk factors which included peripheral intravenous cannulation, intraabdominal surgery, urethral catheterization and intensive care admission. Hospital acquired discitis may be resistant to first-line antibiotic therapy due to the presence of a broader and more complex range of organisms. Broad spectrum antibiotics are then utilized for treatment, thus potentially leading to the evolution of antibiotic resistance.24
Several studies on intravenous drug abusers diagnosed with primary pyogenic spondylodiscitis have been published. A retrospective study done by Ziu et al. found that up to 62% of patients diagnosed with primary pyogenic spinal infections had a history of intravenous drug abuse. These patients commonly present with persistent axial pain. MRI investigations showed the presence of spondylodiscitis (74.5%). Findings of an epidural abscess were occasionally found (18.6%) on MRI evaluation of patients diagnosed with spondylodiscitis. Epidural abscess with no associated spondylodiscitis occurred in only 6.9% of patients. Neurologic deficits were more commonly encountered in patients having epidural abscess on MRI. Majority of the lesions were found to occur in the lumbar spine (57.8%). Single level involvement (69.6%) was more common than multiple segment involvement (30.3%).25 Wang et al. prospectively compared primary pyogenic spinal infections in intravenous drug abusers and non-intravenous drug user patients. Their analysis found that the intravenous drug users had a shorter mean duration of axial pain (51 days versus 105 days) compared to non-intravenous drug users. Further, the mean duration of neurological deficits was lower in intravenous drug abusers (7 days) compared to non-intravenous drug users (12 days). In contrast to the study by Ziu et al., Wang et al. found that the cervical spine was more commonly affected (78%) than the lumbar spine (13%) in intravenous drug users diagnosed with primary spinal infections.26
Propionibacterium acnes, an anaerobic organism found on the skin, has been implicated in certain studies as an opportunistic pathogen that causes spinal infections. Uçkay et al. found that patients typically present with back pain but are generally afebrile. Common risk factors for patients that developed spondylodiscitis secondary to Propionibacterium infection are history of previous surgery (97%) and the presence of instrumentation/implants (76%).27 The diagnosis of spondylodiscitis can be complicated by findings of Modic type I changes on MRI. This can become a source of confusion and lead to the delay in diagnosis and management of patients that present with nonspecific back pain that may potentially have spondylodiscitis. An occult discitis has been described to occur secondary to Propionibacterium acnes. A study by Dudli et al. tested the ability of Propionibacterium acnes to proliferate within intervertebral discs of rat tail models and cause reactive changes in the adjacent bone marrow. Based on their results, the proliferation of Propionibacterium acnes produced disc degeneration, fibrotic endplate erosion and enhanced immunoreactivity. Discs were noted to be hypointense on T2 and hypo or isointense on T1. Endplate regions were said to be hyperintense on T2. The bone marrow was observed to be hypointense on T1. These findings were found to be consistent with Modic type I changes found on MRI.28
Fever of unknown origin (FUO) was previously defined as fever lasting for 3 or more weeks with temperatures above 38.3°C that remained undiagnosed after a week of intensive hospital testing.29 At present, FUO has been defined as fever at or above 38.3°C for 3 weeks or more that remain undiagnosed after 3 days of in-hospital testing or during 2 or more outpatient visits. FUO can be secondary to infectious, rheumatic/inflammatory, neoplastic or other miscellaneous disorders.30 The approach utilized for diagnosis and evaluation of patients with FUO should be focused and relevant to the clinical syndromic presentation of the patient. FUOs considered to have an infectious etiology would typically present in patients as fever accompanied with chills or night sweats. Some patients may also present with weight loss without loss of appetite. Initial diagnostic evaluation includes complete blood count, erythrocyte sedimentation rate and C-reactive protein, blood cultures, as well as relevant radiographs, CT and MRI scans. Focused evaluation entails more detailed history and physical examination, supplemented by an additional battery of tests such as antinuclear antibodies, rheumatoid factor, serum protein electrophoresis, etc. The focused evaluation basically confirms or eliminates the possible differential diagnoses. In some cases, the cause of FUO may still not be established after going through focused evaluation. At this point special tests or tissue biopsies may be employed in order to establish a definitive diagnosis.31
INVESTIGATIONS
Laboratory/Pathology
Hematologic/inflammatory markers
Initial screening of spondylodiscitis includes monitoring blood parameters such as white blood cell count (WBC), erythrocyte sedimentation rate, and C-reactive protein. The WBC may be elevated in 35% of patients. This rarely exceeds 12000 cells/mm3. Elevation of both ESR and CRP (more than 40 mm/h) is seen in patients with spondylodiscitis. Although these are non-specific parameters, both are useful in following the course of treatment for the disease.1,32
Cultures and histology
Cultures should be taken from blood, urine and other possible foci of infection. Collection of cultures allows for determining the choice of antimicrobial therapy to be initiated. Blood cultures are said to be positive in roughly 50% of patients with pyogenic spondylodiscitis.33,34 Some authors recommend that at least 2 to 3 pairs of blood cultures be taken.14
In the event of negative blood cultures, more invasive culture techniques can be performed. The diagnostic yield of biopsies ranges from 47 to 100%.4,17 Direct cultures from the vertebral body or disc can be obtained with a percutaneous needle or an open biopsy.4,5 Image guided percutaneous biopsy is highly specific and is able to predict the presence of spontaneous spondylodiscitis, yet has only moderate accuracy for ruling out the disease. A systematic review and meta-analysis of the diagnostic accuracy of image guided spinal biopsy by Pupaibool et al., They revealed a sensitivity of 52.2% and a specificity of 99.9%. The study yielded that image guided biopsy of the spine had a diagnostic odds ratio of 45.5, a likelihood ratio of a positive test of 16.8 and a likelihood ratio of a negative test of 0.4.35
Open surgical biopsy can be utilized if there is insufficient tissue material obtained by percutaneous needle biopsy. Open surgical biopsy allows for a greater diagnostic yield.5,36 Biopsy material is then sent out for aerobic, anaerobic, fungal and mycobacterial cultures to determine the specific pathogen causing the infectious process. Tissue material can also be processed for histologic analysis. This is a valuable adjunct, not only in distinguishing the various forms of infectious spondylodiscitis, but also in ruling out malignancy.37
Radiology
X-rays
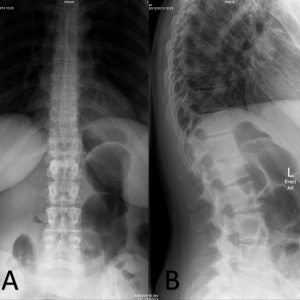
Radiographs are requested as initial screening for the evaluation of pyogenic spondylodiscitis. This can be somewhat unreliable due to its low sensitivity (57%), especially in the early stages of spondylodiscitis.38,39 Early findings include subchondral radiolucency, irregularity of endplates and loss of disc height. Later radiographic changes noted are destruction of adjacent/opposite endplates, loss of vertebral height and development of paravertebral mass (Fig. 3-1A and B).5,40
Computed tomography
Computed tomography (CT) has superior anatomic resolution compared to radiography, hence greater sensitivity in detecting spondylodiscitis. It is an excellent modality for detection of bony abnormalities, such as early endplate destruction, sequestrum or involucrum.5 Plain CT allows for the evaluation of bone changes and detection of gas and calcifications. Contrast enhanced CT can identify for the presence of para-spinal or epidural soft tissue involvement.40,41 CT is used for image guided biopsies of the spine.4,5
Magnetic resonance imaging
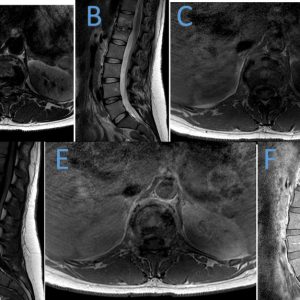
Magnetic resonance imaging (MRI) is the imaging modality of choice due to its high sensitivity (96%), specificity (92%), and accuracy (94%).5,39,41 MRI detects bone edema, disc inflammation, paraspinal and epidural soft tissue involvement, neural compression, and spread into the intradural space. Classic imaging findings specific to pyogenic spondylodiscitis can be found on MRI. Disc space changes include T2 hyperintensity, enhancement or loss of disc height. Adjacent vertebral bodies may develop endplate destruction, and T1 hypointensity and/or T2 hyperintensity. Ill-defined inflammation or swelling and abscess formation may be seen within the paraspinal tissues. The epidural space may develop reactive enhancement and presence of venous plexus distention, phlegmon, or abscess.40 Pyogenic spondylodiscitis typically affects the lumbar spine with occasional adjacent vertebral involvement. Lesions start in the anterior portion of the affected vertebral body. Damage to the disc can be identified in the early stages of the disease process. The rich network of intraosseous anastomoses and profuse capillary network adjacent to the vertebral bodies and discs increases the propensity to infect them. The posterior elements of the spine are relatively less commonly infected compared to the vertebral bodies and intervertebral discs (Fig. 3-2).40,41
Radionuclide imaging studies
Various tracers have been employed to aid in the radiologic identification of spondylodiscitis. Technetium-99m bone scintigraphy is able to detect spondylodiscitis in the first 1 to 2 days of infection. It has a reported sensitivity of 90% and specificity of 78%. Technetium-99m scintigraphy is known to produce false positive results due to the presence of degenerative changes in some patients.1,5,39 Gallium-67 scintigraphy coupled with bone scan have a sensitivity of 90%, specificity of 100%, and accuracy of 100%.5,39 Indium-111-leukocyte scanning is not of particular use due to the poor sensitivity. Spondylodiscitis pathologies are said to present as non-specific photopenic regions with this modality.5,42
Flourine-18 fluorodeoxyglucose positron emission tomography (F-18 FDG PET) has shown promise due to its high sensitivity to spondylodiscitis. This allows for accurate differentiation between degenerative changes and initial findings of spondylodiscitis. The advantages of using this modality include rapid imaging and relatively low exposure to radiation. The major pitfall with FDG PET is its poor ability to distinguish between malignancies and infectious processes occurring in the spine.1,5,14,43,44
DIFFERENTIAL DIAGNOSES
Any patient considered to have pyogenic spondylodiscitis should be worked up to rule out other disease processes. Differential diagnoses include degenerative changes, neoplastic spinal disease, trauma to the spine in the form of vertebral compression fractures, inflammatory spondyloarthropathies, and tuberculous processes.45
Tumors of the spine may be mistaken for an infectious process on imaging. The two can be differentiated with the presence or absence of disc involvement. Neoplasms typically involve the vertebrae and spare the intervertebral disc. This is due to the presence of the cartilaginous endplate that separates the disc from the vertebrae. Malignant neoplasms may mimic pyogenic spondylodiscitis if there are perforations in the endplate, thus leading to tumor invasion and subsequent involvement of the intervertebral disc.1,46,47
Degenerative changes of the spine should be distinguished from infectious spondylodiscitis. This may present as disc herniation associated with disc space collapse, dessication, bulge, end-plate erosion, or annular tear on MRI and osteoporosis with vertebral collapse.1 Specifically, Modic Type 1 endplate changes are imaging findings on MRI that can be confused with spondylodiscitis. This may appear as T2 hyperintensity along the vertebral endplates adjacent to a degenerating disc. Use of gadolinium contrast may also produce enhancement of both the endplates and disc.48
MANAGEMENT
The goals of treatment of spontaneous pyogenic spondylodiscitis are to relieve pain, prevent and/or reverse neurologic deficits, eradicate infection, prevent relapse, and establish spine stability.1,5 These goals can be achieved through conservative and surgical means.
Conservative Treatment
Antibiotic therapy
Ideally, antibiotics are started once cultures or biopsies have been collected and the specific pathogen has been identified. This allows for targeted therapy of the causative organism. Intravenous antibiotics are usually given 4 to 6 weeks until clinical improvement is noted and infectious parameters (ESR and CRP) have been reduced. An additional course of oral antibiotics is then administered for another 6 weeks.18,49 Pyogenic spondylodiscitis can be considered a chronic infection because of delays in presentation and diagnosis of this disease. Due to its chronicity, antimicrobial therapy should be given to patients for at least 12 weeks.50 This has been recently queried by a randomized clinical trial done in France. The study aimed to establish whether a 6-week course of antibiotic therapy is non-inferior to a 12 week course of antimicrobial treatment in patients diagnosed with pyogenic vertebral osteomyelitis. Clinical cure was achieved in both treatment groups (90.9% of patients with 6 week treatment regimen versus 90.9% of patients with 12 week treatment regimen). Antibiotic intolerance was noted to occur in 7% of patients in the 6 week treatment group versus 5% of patients in the 12 week treatment group. The study concluded that a 12 week course had no clinical advantage over the 6 week antibiotic regimen.51
The role of adjuvant antibiotic therapy for spondylodiscitis has not yet been established. The use of fusidic acid in combination with penicillin was shown to have lower recurrence rates (5% versus 20%) compared to beta-lactam monotherapy.52 Another antibiotic used in combination therapy is rifampicin. Adjunctive use of rifampicin was noted to provide benefit in the treatment of prosthetic and bone infections.53 A study by Viale et al. showed that combination use of high dose levofloxacin supported by rifampicin had high response rates among patients with pyogenic spondylodiscitis (96%)(Table 3-2).54
| Pathogen | Antibiotic Therapy |
|---|---|
| S. aureus | Oxacillin/Flucloxacillin/Ceftriaxone (MSSA) Vancomycin/Teicoplanin (MRSA) |
| Streptococcus | Amoxicillin/Benzylpenicillin/Ceftriaxone |
| Pseudomonas | Ceftazidime/Meropenem/Ciprofloxacin |
| Enterococci | Amoxicillin |
| Gram negative organisms | Ceftriaxone/Cefotaxime |
| Anaerobes | Metronidazole/Clindamycin |
Immobilization
Immobilization and bracing were advised for the treatment of intractable pain in patients with spondylodiscitis. These techniques were also advocated for addressing instability due to vertebral destruction secondary to the infectious process. Besides addressing instability, bracing also allows for prevention of further deformity of the spine. Some authors recommend bed rest with low-molecular weight heparin treatment for 3 to 4 weeks, as well as use of orthoses for 1 to 3 months. In general, patients are allowed to mobilize with a thoracolumbosacral orthoses (TLSO) as tolerated.10,50,55,56
Surgical Treatment
Pyogenic spondylodiscitis is most often managed conservatively. This entails administration of intravenous followed by oral antibiotics for eradication of infection and the use of immobilization of the spine for pain control. Only 10 to 20% of patients diagnosed with pyogenic spondylodiscitis eventually undergo surgical management.56 Indications for surgical management of pyogenic spondylodiscitis include failure of conservative treatment, severe kyphosis, neural element compression, and spinal instability secondary to osteolysis.1,5,14,57 Emergent surgical decompression is required for patients presenting with spinal cord or cauda equina compression as the prognosis for neurologic recovery is better compared to non-operative management.58
The standard procedure for surgical treatment of pyogenic spondylodiscitis is anterior decompression and debridement, coupled with stabilization of the spine. Most authors recommend utilizing an anterior approach for exposure of the involved segment of the spine. This is due to the fact that pyogenic spondylodiscitis commonly affects the anterior vertebral elements. The laminae, pedicles and facets are spared, thus preventing any further subluxation and instability. In the lumbar spine, a retroperitoneal approach is utilized, as a transperitoneal approach runs the risk of potentially seeding the peritoneal cavity. Extensive debridement of the infected vertebrae and discs is performed until healthy bone is encountered. The defect left after debridement is then spanned through the use of autologous bone grafts. Interbody or titanium cages can also be used. These methods allow for spanning interposition, reconstruction, and ultimately fusion of the involved segment of the spine.1,5,58
Most of the literature still advocates extended periods of bed rest after surgery for fear of using spinal instrumentation as a new nidus of infection may form on the metal hardware secondary to biofilm. Some reports, on the other hand, recommended supplementing anterior debridement and fusion with spinal fixation to allow for early mobilization, prevent postoperative kyphotic deformity and lower rates of pseudoarthroses.14,58 Instrumentation is considered in order to address instability of the involved spinal segment in patients with pyogenic spondylodiscitis. This can be assessed preoperatively with the presence of severe destruction of the vertebral body and disc. Intraoperative instability can be expected after radical debridement and resection of the infected spinal elements.59 A retrospective study of 101 patients with pyogenic spondylodiscitis showed that corpectomy, fusion, and posterior instrumentation had a higher success rate compared to corpectomy and fusion alone (100% vs 25%).10 Another retrospective study compared anterior debridement plus interbody fusion versus combined anterior debridement and fusion with posterior instrumented fusion in patients with lumbar pyogenic spondylodiscitis. Both groups had high fusion rates without any recurrence of infection, although both exhibited reduction of intervertebral height and loss of sagittal profile on follow-up evaluation.60
Other surgical procedures for the treatment of pyogenic spondylodiscitis may be utilized in certain situations. Laminectomy is only indicated for primary spinal epidural abscess, as this may cause further instability of the spine. Secondary epidural abscesses with pus extending to the posterior may be addressed by laminectomy supplemented by posterior instrumentation, with or without staged anterior reconstruction. Another procedure that can be used for the treatment of pyogenic spondylodiscitis is percutaneous transpedicular discectomy. This is said to be an excellent treatment option for patients with early uncomplicated spondylodiscitis.10,61 Percutaneous posterior methods have also been used as an alternative to the traditional form of treatment for patients with pyogenic spondylodiscitis that have multiple morbidities. When compared to the standard form of treatment, these percutaneous methods had similar mortality, relapse and failure rates but with better clinical outcomes.56
Early complications may occur after surgery. These include wound infections, sepsis, pleural effusion, pulmonary embolism, cerebrospinal fluid fistula, ileus, ureteral damage, pneumonia, graft fracture and progressive neurologic deficit. Late onset complications include graft resorption and fracture, non-union, and progressive kyphosis and persistent pain.1
PROGNOSIS & OUTCOME
The mortality rate for pyogenic spondylodiscitis ranges from 0 to 11%.4,5,45 In a Danish study, long term mortality was increased in patients with comorbidities such as infectious, neoplastic, endocrine, cardiovascular, respiratory, gastrointestinal, musculoskeletal, genitourinary and alcohol and drug abuse-related diseases.62 Residual symptoms occur with either conservative or surgical management of patients with pyogenic spondylodiscitis secondary to destruction and degeneration of the involved spinal segments.14 Residual back pain was noted in patients treated conservatively (64%) and surgically (26%).10 Disability occurs in a third of patients secondary to residual neurologic deficits and intractable pain.5 Recurrence of symptoms was said to occur within 6 months after completion of antibiotic therapy.45 One study reported of having a relapse rate of 11.8%. Relapse of spondylodiscitis was associated with severe vertebral destruction, recurrent bacteremia, paravertebral abscesses and chronic draining sinuses.36
CONCLUSION
Spontaneous pyogenic spondylodiscitis is a rare affliction of the spine. Although uncommon, its incidence has begun to rise due to the increase in number of the elderly population who are susceptible to the disease. This is associated with the presence of multiple co-morbidities making the elderly more to prone to have the disease. The incidence of spontaneous pyogenic spondylodiscitis has also increased secondary to the availability of more effective diagnostic modalities. Due to the insidious onset of this disease, a high index of suspicion is needed for early diagnosis and treatment. Timely detection and management permits for improved long term outcomes for patients with pyogenic spondylodiscitis. MRI is still the gold standard imaging modality for detecting pyogenic spondylodiscitis. Microbiological diagnosis is achieved through the use of cultures and biopsies. This is essential for the administration of targeted antibiotic therapy, which is the cornerstone of treatment of this disease. Immobilization and bracing addresses pain, instability and deformity of the spine. Surgery is reserved for patients who have undergone failed conservative treatment, neurologic deterioration and spinal instability.
REFERENCES
- Skaf GS, Domloj NT, Fehlings MG, et al. Pyogenic spondylodiscitis: an overview. J Infect Public Health. 2010;3(1):5-16.
- Govender S. Spinal infections. J Bone Joint Surg Br. 2005;87(11):1454-1458.
- Nathoo N, Caris EC, Wiener JA, Mendel E. History of the vertebral venous plexus and the significant contributions of Breschet and Batson. Neurosurgery. 2011;69(5):1007-1014.
- Fantoni M, Trecarichi EM, Rossi B, et al. Epidemiological and clinical features of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16 Suppl 2:2-7.
- Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65 Suppl 3:iii11-24.
- Sur A, Tsang K, Brown M, Tzerakis N. Management of adult spontaneous spondylodiscitis and its rising incidence. Ann R Coll Surg Engl. 2015;97(6):451-455.
- Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79(6):874-880.
- Perronne C, Saba J, Behloul Z, et al. Pyogenic and tuberculous spondylodiskitis (vertebral osteomyelitis) in 80 adult patients. Clin Infect Dis. 1994;19(4):746-750.
- Legrand E, Flipo RM, Guggenbuhl P, et al. Management of nontuberculous infectious discitis. Treatments used in 110 patients admitted to 12 teaching hospitals in France. Joint Bone Spine. 2001;68(6):504-509.
- Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976). 2000;25(13):1668-1679.
- Turner DP, Weston VC, Ispahani P. Streptococcus pneumoniae spinal infection in Nottingham, United Kingdom: not a rare event. Clin Infect Dis. 1999;28(4):873-881.
- Saeed MU, Mariani P, Martin C, et al. Anaerobic spondylodiscitis: case series and systematic review. South Med J. 2005;98(2):144-148.
- Pilmis B, Israel J, Le Monnier A, Mizrahi A. Spondylodiscitis due to anaerobic bacteria about a case of Parvimonas micra infection. Anaerobe. 2015;34:156-157.
- Sobottke R, Seifert H, Fätkenheuer G, Schmidt M, Gossmann A, Eysel P. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008;105(10):181-187.
- Kehrer M, Pedersen C, Jensen TG, Lassen AT. Increasing incidence of pyogenic spondylodiscitis: a 14-year population-based study. J Infect. 2014;68(4):313-320.
- Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis. 1979;1(5):754-776.
- Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39(1):10-17.
- Kapsalaki E, Gatselis N, Stefos A, et al. Spontaneous spondylodiscitis: presentation, risk factors, diagnosis, management, and outcome. Int J Infect Dis. 2009;13(5):564-569.
- Turunc T, Demiroglu YZ, Uncu H, Colakoglu S, Arslan H. A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J Infect. 2007;55(2):158-163.
- Yoon YK, Jo YM, Kwon HH, et al. Differential diagnosis between tuberculous spondylodiscitis and pyogenic spontaneous spondylodiscitis: a multicenter descriptive and comparative study. Spine J. 2015;15(8):1764-1771.
- Pigrau C, Almirante B, Flores X, et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med. 2005;118(11):1287.
- Principi N, Esposito S. Infectious discitis and spondylodiscitis in children. Int J Mol Sci. 2016;17(4):539.
- Khan IA, Vaccaro AR, Zlotolow DA. Management of vertebral diskitis and osteomyelitis. Orthopedics. 1999;22(8):758-765.
- Lillie P, Thaker H, Moss P, et al. Healthcare associated discitis in the era of antimicrobial resistance. J Clin Rheumatol. 2008;14(4):234-237.
- Ziu M, Dengler B, Cordell D, Bartanusz V. Diagnosis and management of primary pyogenic spinal infections in intravenous recreational drug users. Neurosurg Focus. 2014;37(2):E3.
- Wang Z, Lenehan B, Itshayek E, et al. Primary pyogenic infection of the spine in intravenous drug users: a prospective observational study. Spine (Phila Pa 1976). 2012;37(8):685-692.
- Uçkay I, Dinh A, Vauthey L, et al. Spondylodiscitis due to Propionibacterium acnes: report of twenty-nine cases and a review of the literature. Clin Microbiol Infect. 2010;16(4):353-358.
- Dudli S, Liebenberg E, Magnitsky S, Miller S, Demir-Deviren S, Lotz JC. Propionibacterium acnes infected intervertebral discs cause vertebral bone marrow lesions consistent with Modic changes. J Orthop Res. 2016;34(8):1447-1455.
- Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore). 1961;40:1-30.
- Cunha BA. Fever of unknown origin: clinical overview of classic and current concepts. Infect Dis Clin North Am. 2007;21(4):867-915, vii.
- Cunha BA. Fever of unknown origin: focused diagnostic approach based on clinical clues from the history, physical examination, and laboratory tests. Infect Dis Clin North Am. 2007;21(4):1137-1187, xi.
- Rath SA, Neff U, Schneider O, Richter HP. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review of 43 consecutive surgically treated patients. Neurosurgery. 1996;38(5):926-933.
- Di Martino A, Vaccaro AR, Lee JY, Denaro V, Lim MR. Nucleus pulposus replacement: basic science and indications for clinical use. Spine (Phila Pa 1976). 2005;30(16 Suppl):S16-22.
- Lehovsky J. Pyogenic vertebral osteomyelitis/disc infection. Baillieres Best Pract Res Clin Rheumatol. 1999;13(1):59-75.
- Pupaibool J, Vasoo S, Erwin PJ, Murad MH, Berbari EF. The utility of image-guided percutaneous needle aspiration biopsy for the diagnosis of spontaneous vertebral osteomyelitis: a systematic review and meta-analysis. Spine J. 2015;15(1):122-131.
- McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
- Rankine JJ, Barron DA, Robinson P, Millner PA, Dickson RA. Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J. 2004;80(948):607-609.
- Varma R, Lander P, Assaf A. Imaging of pyogenic infectious spondylodiskitis. Radiol Clin North Am. 2001;39(2):203-213.
- Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157(1):157-166.
- Diehn FE. Imaging of spine infection. Radiol Clin North Am. 2012;50(4):777-798.
- Prodi E, Grassi R, Iacobellis F, Cianfoni A. Imaging in spondylodiskitis. Magn Reson Imaging Clin N Am. 2016;24(3):581-600.
- Palestro CJ, Kim CK, Swyer AJ, Vallabhajosula S, Goldsmith SJ. Radionuclide diagnosis of vertebral osteomyelitis: indium-111-leukocyte and technetium-99m-methylene disphosphonate bone scintigraphy. J Nucl Med. 1991;32(10):1861-1865.
- Schmitz A, Risse JH, Grünwald F, Gassel F, Biersack HJ, Schmitt O. Fluorine-18 fluorodeoxyglucose positron emission tomography findings in spondylodiscitis: preliminary results. Eur Spine J. 2001;10(6):534-539.
- Stumpe KD, Zanetti M, Weishaupt D, Hodler J, Boos N, Von Schulthess GK. FDG positron emission tomography for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine detected on MR imaging. AJR Am J Roentgenol. 2002;179(5):1151-1157.
- Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56(6):401-412.
- Gabe MJ, Allmendinger AM, Krauthamer A, Spektor V, Destian S, Zablow B. Imaging manifestations of malignant neoplasia mimicking pyogenic osteodiscitis. Clin Imaging. 2010;34(4):309-315.
- Kakitsubata Y, Theodorou DJ, Theodorou SJ, Nabeshima K, Tamura S. Metastatic disease involving the discovertebral junction of the spine. Joint Bone Spine. 2009;76(1):50-56.
- Longo M, Granata F, Ricciardi GK, Gaeta M, Blandino A. Contrast-enhanced MR imaging with fat suppression in adult-onset septic spondylodiscitis. Eur Radiol. 2003;13(3):626-637.
- Carragee EJ, Kim D, van der Vlugt T, Vittum D. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976). 1997;22(18):2089-2093.
- Grados F, Lescure FX, Senneville E, Flipo RM, Schmit JL, Fardellone P. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Joint Bone Spine. 2007;74(2):133-139.
- Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385(9971):875-882.
- Jensen AG, Espersen F, Skinhøj P, Frimodt-Møller N. Bacteremic Staphylococcus aureus spondylitis. Arch Intern Med. 1998;158(5):509-517.
- Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med. 2008;168(8):805-819.
- Viale P, Furlanut M, Scudeller L, et al. Treatment of pyogenic (non-tuberculous) spondylodiscitis with tailored high-dose levofloxacin plus rifampicin. Int J Antimicrob Agents. 2009;33(4):379-382.
- Veillard E, Guggenbuhl P, Morcet N, et al. Prompt regression of paravertebral and epidural abscesses in patients with pyogenic discitis. Sixteen cases evaluated using magnetic resonance imaging. Joint Bone Spine. 2000;67(3):219-227.
- Rutges JP, Kempen DH, van Dijk M, Oner FC. Outcome of conservative and surgical treatment of pyogenic spondylodiscitis: a systematic literature review. Eur Spine J. 2016;25(4):983-999.
- Gasbarrini AL, Bertoldi E, Mazzetti M, et al. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur Rev Med Pharmacol Sci. 2005;9(1):53-66.
- Cheung WY, Luk KD. Pyogenic spondylitis. Int Orthop. 2012;36(2):397-404.
- Rayes M, Colen CB, Bahgat DA, et al. Safety of instrumentation in patients with spinal infection. J Neurosurg Spine. 2010;12(6):647-659.
- Ha KY, Shin JH, Kim KW, Na KH. The fate of anterior autogenous bone graft after anterior radical surgery with or without posterior instrumentation in the treatment of pyogenic lumbar spondylodiscitis. Spine (Phila Pa 1976). 2007;32(17):1856-1864.
- Hadjipavlou AG, Katonis PK, Gaitanis IN, Muffoletto AJ, Tzermiadianos MN, Crow W. Percutaneous transpedicular discectomy and drainage in pyogenic spondylodiscitis. Eur Spine J. 2004;13(8):707-713.
- Aagaard T, Roed C, Dahl B, Obel N. Long-term prognosis and causes of death after spondylodiscitis: a Danish nationwide cohort study. Infect Dis (Lond). 2016;48(3):201-208.