Bryan Rynearson and James D. Kang
BIOLOGY OF THE DEGENERATIVE INTERVERTEBRAL DISC
Biologic therapies are highly targeted approaches to treating degeneration of the intervertebral disc (IVD). The pathophysiology of the degenerative process is complex and likely multifactorial in nature. Factors such as age, excessive mechanical loading, environmental exposures, and genetic predispositions may all be important contributing factors.
The degenerative cascade is well-described and is hallmarked by the loss of homeostasis of the extracellular matrix (ECM) resulting in a progressive derangement of composition.1 There is a gradual loss of functional IVD cells,2 down regulation of proteoglycan synthesis, elevated levels of catabolic enzymes,3 upregulation of inflammatory cytokines4 and accumulation of products of oxidative stress5 within the nucleus pulposus (NP).
These biochemical changes may manifest with progressive loss of disc height and impaired biomechanical properties of the disc which may impact the entire functional spinal unit and adjacent segments. Once altered, the aberrant disc mechanics lead to abnormal loading of the affected disc and further perpetuate degenerative changes.6
The inciting factor of IVD degeneration is unknown, but growing evidence suggests an altered phenotype that results from aging of the IVD cells, a process known as cellular senescence, is an important contributor. Compared to nucleus pulposus cells obtained from healthy discs, degenerated cells exhibit a shorter mean telomere length, increased expression of senescence associated beta-galactosidase (SA-beta-gal) and reduced replicative potential7,8 typical of an aging cell. Degenerated cells compared to healthy cells also express significantly higher levels of tumor suppressor genes involved in the activation of irreversible cellular growth arrest.8
Many factors contribute to intervertebral disc degeneration including genetics, biochemical changes and biomechanical loading. Degenerated discs may or may not be painful. Painful discs may contain cells that express a higher concentration of pro-inflammatory cytokines or pain-modulating molecules.
For these reasons, the goals of biologic therapies are the reestablishment of normal ECM homeostasis of the IVD and decreasing IVD-associated pain. Current approaches include intradiscal injection of growth factors or pro-inflammatory cytokine antagonists, gene therapy, cell-based therapy and antioxidant agents. Molecular therapies including growth factors and anti-inflammatory agents will be covered in another chapter, and therefore, this chapter will focus on gene therapy, cell-based therapy and briefly on antioxidants.
GENE THERAPY
Background
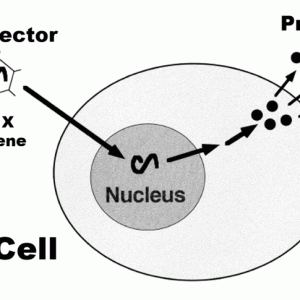
Gene therapy involves altering the gene expression of native IVD cells either by upregulating production of specific architectural proteins, inhibitors of catabolic enzymes or a combination of both. This effect is produced through the use of specialized vectors to deliver a desired gene or genes to target cells. Once “infected” or transduced, cells use their molecular machinery to synthesize and translate the corresponding messenger RNA into the desired protein (Fig. 6-1). This is referred to as transgenic expression. Currently, most genes selected are growth factors due to studies that demonstrated exogenously administered growth factors upregulate production of target ECM proteins in the IVD which become deficient during the degenerative process. Unfortunately, the effects of protein therapy proved short-lived necessitating a different longer acting approach.9
Vectors
Gene vector systems are of two main types: viral or non-viral. Non-viral systems are plasmid-based, do not possess pathogenicity and are relatively inexpensive to synthesize.10 The primary concern when employing non-viral vector systems is the poor vector delivery owing to the relative impermeability of target cell membranes to large molecules such as plasmids.11 Additionally, genes transferred via non-viral vectors integrate poorly into the host cell genome and exist as extrachromosomal or episomal genes. The episomal genetic material tends not to be replicated and transferred to daughter cells and typically leads to a decline in gene expression over time.1 This decline in gene expression confers obvious limitations in the management of chronic diseases such as IVD degeneration.
However, there has been some success in the development of a delivery system to prolong transgene expression using a non-viral vector system for IVD degeneration therapy. Nishida et al. demonstrated that NP cells in vivo transfected with the addition of ultrasound with microbubbles significantly enhances transfection efficiency of plasmid DNA, reporting ongoing expression up to 24 weeks.11 In this technique, the plasmids are incorporated within the microbubbles. Ultrasound application serves two purposes. First, it is believed to create small pores on the surface of target cells, a process termed sonoporation. Second, application of ultrasound causes the microbubbles to burst which releases the plasmids into the intracellular environment. This technique is regarded as safe; however, this particular study did not assess for cytotoxic effects.
In contrast, viral vectors confer unique advantages and disadvantages. They are more expensive than their non-viral counterparts as they require more specialized facilities to synthesize. They also carry the potential to cause host inflammatory reactions and clinically apparent deleterious effects depending on the viral vector, dosing, and location of administration. Perhaps the most important difference between viral and non-viral vectors, however, is that viral vectors are more efficient at transgene expression. Relatively few viral vectors are needed to accomplish a given level of protein expression in the target cell population. It is for this reason principally that viral vectors are the most common system used in gene therapy for the degeneration of the IVD.
Viral vectors are heterogeneous and can be subdivided into genome incorporating retrovirus type (retrovirus, lentivirus) and non-genome incorporating plasmid type (herpes, adeno, and adeno-associated viruses).9 Depending on the gene of interest, the disease being treated and the target cell, any of these vectors are reasonable options. Currently, adeno-associated virus (AAV) is the most widely used viral vector in the study of intervertebral disc degeneration (IDD).12 Though not as effective as its adenovirus predecessor regarding the magnitude of gene expression after transduction, AAV exhibits many beneficial properties such as non-pathogenicity, site-selective integration with stable gene expression and the ability to infect quiescent non-dividing cells making them what many consider ideal for the study of IVD disease.13
Feasibility
Numerous animal studies have demonstrated the ability of viral vectors to transduce IVD cells in the in vitro and in vivo environments. The AAV vector can successfully transduce human NP cells in vitro and rabbit NP cells in vivo with the luciferase and beta-galactosidase (LacZ) marker genes.14 Moon et al. had similar success in transducing human NP cells surgically obtained from degenerated discs in vitro with the LacZ gene using adenovirus (ad/CMV-lac).15 They also reported the ability of the vector to transduce cells regardless of the grade of disc degeneration.
Transforming growth factor beta (TGF-B) is one of numerous growth factors believed to have a regulatory role in the maintenance of the ECM of the intervertebral disc and as such has garnered interest as a therapeutic target in degenerative disc disease. In vivo transduction of TGF-B via adenovirus vector was shown to result in a 30-fold increase in the active form of the growth factor and produced a 200% increase in proteoglycan synthesis.16 Bone morphogenetic protein (BMP) is another family of growth factors of therapeutic interest. Similar to TGF-B, many of the BMP members are believed to be involved in the regulation of important proteins of the disc ECM. This was supported by observations after in vivo administration of adenovirus-vectored LIM mineralization protein 1 (ad-LMP1), a known upstream regulator of several BMP genes, produced significant increases in expression of BMP-2, BMP-7, aggrecan, and glycosaminoglycans.17
Efficacy
Although less abundant in the literature than feasibility studies, important in vivo data has demonstrated the capacity of viral vectored genes to potentially alter the course of degeneration within the IVD. Administration of AAV serotype 2 (AAV2) carrying BMP-2 or tissue inhibitor of metalloproteinase 1 (TIMP-1) transgene was utilized in a needle puncture annulotomy model of IVD degeneration in the lumbar spine of rabbits.18 TIMP-1 was chosen as it is a naturally occurring endogenous inhibitor of the catabolic enzyme MMP-1 found in the extracellular environment. All treated discs in this study underwent degenerative changes to some degree. However, beneficial effects were observed at both the histologic and macroscopic level in both treatment groups at the end of the study at 12 weeks. Compared to untreated injured controls, treatment groups demonstrated an attenuated fibrotic response, higher cellularity and overall a relatively maintained architecture. Serial MRI scans of treated levels showed significantly better maintenance of NP area and signal intensity.
Viscoelastic analysis during axial loading which is largely influenced by collagen integrity was also performed. This analysis revealed that AAV2-BMP2 treated discs had properties more closely related to control rather than injured non-treated discs.18 The viscoelastic properties of the AAV2-TIMP1 treated discs more closely resembled that of untreated injured controls. They did not report any adverse events attributable to the injection.
In a rabbit model, similar to the aforementioned, cells were transduced with adenovirus carrying the Sox-9 gene, which is a known regulator of type 2 collagen synthesis, and injected into the IVD after disc injury. Histologic analysis demonstrated treated discs better maintained their chondrocytic appearance of the NP cells and had a more normal NP architecture compared with degenerated untreated controls at the study conclusion at 5 weeks.19 There was one perioperative death in this study.
Safety and Special Considerations
Use of viral vectors deserves special scrutiny owing to the immunogenic potential of these agents and the close anatomic proximity of the injection site to vital neural structures and the cerebrospinal fluid.
Animal models show that purposeful injection of AAV carrying anabolic cytokines such as TGF-B and BMP2 into the epidural space do not produce clinical, histological or biochemical abnormalities even at high doses.20 Conversely, adenovirus carrying these same genes were associated with serious signs of toxicity, especially at higher doses. These included diminished hind limb sensation, flaccid paralysis and even death.20 Histologic studies of these animals confirmed extensive areas of inflammation and signs of spinal cord infarction.
This study also demonstrated that an immunogenic response was associated with the degree of expression of the delivered gene and that the high degree of vascularity in the epidural space may attenuate the ability of a vector to reach its target before being removed by the circulatory system. The researchers concluded that adenovirus’s superior rate of cellular transduction likely accounted for the significantly different side effects seen compared to the AAV group.
The local effects within the disc after exposure to AAV has also been studied. It appears there is no significant immunogenic response after injection into the IVD even after repeat maintenance dosing which supports the idea that the IVD may be an immune privileged site.9 However, a significant humoral response is generated after exposure in AAV-naïve subjects. In one study this response was shown to significantly attenuate but not prevent transgene expression when animal subjects were pre-exposed to the virus.14 Though this does not appear to affect clinical safety, this finding has important potential clinical significance as over 90% of people older than age 50 exhibit AAV antibodies21 that may impact treatment response.
Final Thoughts
Gene therapy has demonstrated potential to affect IDD positively. However, the current supporting literature has important limitations which include small sample sizes, unknown duration of transgenic expression, and, perhaps most importantly, to our knowledge, a lack of data examining the impact gene therapy may have on clinical behaviors reflective of pain and disability in the setting of IDD. Furthermore, it is still unclear if current gene therapy interventions can produce regeneration of the IVD, i.e. reverse the degenerative process and not just slow its course. This may affect the timeframe in which this therapy can be used. Finally, there remain important safety considerations which need to be carefully addressed before gene therapy is ready for human trials. One such consideration involves the use of an inducible system for post hoc regulation of transgene expression.22
CELL-BASED THERAPY
Background
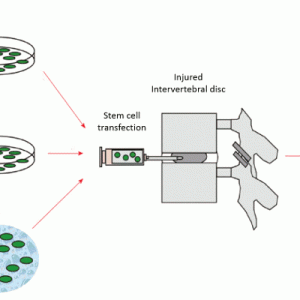
These therapies are focused on direct repopulation of healthy cells in the degenerated disc environment. Cell-based therapy commonly employs adult-derived mesenchymal stem cells from sources that include bone marrow, human umbilical cord blood, adipose and skeletal muscle. These cells can be implanted in their undifferentiated state or differentiated to the desired phenotype in vitro before implantation and can be imbedded with or without a carrier matrix (Fig. 6-2). Cell-based therapy is an attractive alternative modality to gene therapy because it addresses the issue of a diminished native cell population.
Early cell-based research strategies focused on the use of host IVD cells to treat IDD. Benefits of this method over the use of allogeneic stem cells include less time and cost in cell procurement and preparation as it obviates the risk of immunogenic response and the need for screening a donor for transmissible diseases. Indeed, data supported re-implantation of autologous NP cells could delay degenerative changes in the IVD in animal models.23,24
However, important drawbacks to autologous disc cell re-implantation exist. Degenerative NP cells exhibit limited in vitro expansion, and there is a significant limitation on the volume of donor tissue that can be obtained per disc. Furthermore, the extraction of the disc cells has been shown to exacerbate degenerative changes in healthy donor discs in which no signs of degeneration existed previously.25 It is for these reasons that stem cells are the primary type of cell-based therapy studied today.
Feasibility
The pluripotent nature of mesenchymal stem cells (MSCs) has been demonstrated in numerous tissue systems making them a desirable treatment modality for IDD. Adult-derived MSCs have been shown to possess the ability to differentiate into the chrondrocyte-like cells that populate the intervertebral disc. Bone marrow-derived MSCs have demonstrated the ability to express constituent ECM proteins such as aggrecan, decorin, fibromodulin and cartilage oligomeric matrix protein genes consistent with the differentiated chondroid cell phenotype of the IVD.26 Furthermore, the relative ratio of these synthesized proteoglycans is consistent with that of native NP cells.27
Direct cell-cell interactions appear to play a crucial role in the differentiation of MSCs into chondrocyte-like cells. One study demonstrated that adult-derived MSCs from bone marrow, when co-cultured with NP cells obtained from degenerated IVDs, produced phenotypic changes in both cell types. When co-cultured with NP cells, MSCs upregulate expression of Sox-9, a transcription factor expressed during chondrocyte differentiation, as well as type VI collagen and the proteoglycan versican. Similarly, the degenerated NP cells in co-culture with MSCs also upregulated expression of Sox-9 as well as collagen and proteoglycan synthesis to the level of their non-degenerated counterparts.28 Sobajima et al. demonstrated an ideal ratio of NP to MSCs regarding maximizing GAG production. Nucleus pulposus cell to MSC ratios of 75:25 and 50:50 produced more than twice the GAG than NP culture alone.29 Using murine muscle-derived stem cells, Vadala et al. similarly showed that an NP to MSC ratio of 75:25 produced the highest amount of GAG.30 Furthermore, DNA analysis in this study revealed a three- to four-fold increase in cells over either NPC or MSC culture alone.
After in vitro differentiation, MSCs from human umbilical tissue have been successfully injected into explanted rabbit IVDs and remain viable for at least four weeks while demonstrating continued ability to maintain ECM rich in both proteoglycans and type 2 collagen.31
Efficacy
In the rabbit disc injury model, autologous differentiated bone marrow-derived MSCs have shown the potential to slow the degenerative process in vivo. This has been evidenced by maintenance of disc height, MRI signal and nuclear ECM regarding both histologic appearance and protein composition in treated injured discs after 24 weeks compared to untreated injured controls.32 Another study showed undifferentiated MSCs from human umbilical tissue produced similar findings of attenuated degeneration regarding MRI signal and histologic analyses.33 Also, treated discs in this study demonstrated improved biomechanical properties compared to untreated injured controls. Neither of these studies reported any deaths or other adverse clinical events.
Ahn et al. also examined the effect of implanted human umbilical cord blood-derived MSCs obtained from four different donors.34 They employed the same annulotomy induced degeneration model in rabbits as previously described. Before implantation, the expression of two different cell surface receptors believed to be involved in cellular differentiation pathways was quantified, transforming growth factor-beta receptor I/activin-like kinase receptor 5 (TβRI/ALK5) and TβRII. Receptor expression was categorized as low, medium or high. The MSCs were embedded in a hyaluronic acid gel carrier matrix and injected at three weeks post-injury after MRI had confirmed signs of degeneration.
Pfirrmann and histologic degeneration grades were determined at twelve weeks and were found to be lowest in the high receptor expression plus carrier group compared to all other treatment groups indicating the least amount of degeneration in this cohort. Pfirrmann scores are calculated using disc height, disc signal intensity and disc signal homogeneity from MRI. In addition to these beneficial findings, this study suggests that the potential for MSCs obtained from the same tissue type to undergo chondrogenesis is not equivalent between all donors, and variations in cell receptor expression might account for these differences.
Human Clinical Trials
There are important human clinical trials currently underway employing stem cells in patients with lumbar IDD. One such trial, a prospective, multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of a single percutaneous intradiscal injection of Rexlemestrocel-L. This compound is comprised of adipose-derived allogenic mesenchymal precursor cells (MPCs) embedded in a hyaluronic acid (HA) carrier gel and was studied in 100 patients suffering from chronic low back pain (>6 months symptoms) recalcitrant to conservative therapies (>3 months of therapy). Data from this trial suggests numerous statistically significant benefits over controls (saline or HA injections). Approximately half of patients had at least 50% reduction in back pain at both 6 and 12 months compared to 22.2% and 12.5% for HA and saline groups, respectively.35 Additionally, 50% of patients demonstrated at least a 15-point improvement in functional score on the Oswestry Disability Index (ODI) at both 6 and 12 months compared to 31.6% and 17.7% for HA and saline groups, respectively. Finally, the need for additional intervention following injection (i.e. epidural steroid injection or surgery) was also examined. Depending on the concentration of MPC used, secondary intervention rates ranged from only 3.3% to 6.9% in the treatment groups compared to 15% and 25% for HA and saline control groups, respectively. As of early 2016, Rexlemestrocel-L is recruiting participants for its phase 3 trial.
Another noteworthy human clinical trial involves single percutaneous lumbar disc injection of allogenic juvenile cultured chondrocytes in a fibrin matrix (NuQu) in a similar patient population as the aforementioned clinical trial. Phase 1 efficacy and safety data in 15 patients suggests numerous beneficial effects of this therapy. There was a statistically significant improvement in multiple pain and function indices (ODI, NRS, and SF-36).36 Additionally, MRI imaging demonstrated improvement in disc characteristics in 77% of patients by 12 months post injection. These changes ranged from increased disc height and contour to decreased size or even complete resolution of annular tears. No side effects were reported, but 20% of patients went on to receive total disc replacements for persistent low back pain. NuQu was projected to finish its phase 2 multicenter trial in 44 patients in September 2016.
Safety and Special Considerations
Multiple studies have reported no discernible adverse clinical effects in animals after injections of MSCs into the disc.29,32-34 Histologic analyses also suggest MSC injection is not associated with neovascularization or an inflammatory response.29,34 However, reports of untoward effects following MSC injection into the IVD exist. Vadala et al. administered allogenic bone marrow-derived MSCs without a carrier matrix three weeks after needle annulotomy in a rabbit model and noted significant osteophyte formation at all injected disc levels as early as nine weeks.37 The osteophyte location was adjacent to the MSC injection site which was contralateral to the site of needle puncture. Histologic and immunofluorescent analyses revealed the presence of the MSCs within the osteophytes ultimately leading the researchers to conclude that the swelling pressure within the disc during MSC injection might have resulted in leakage of the cells from the discs into the adjacent tissues. The cells likely underwent chondrogenic differentiation followed by endochondral ossification and osteophyte formation. Though the majority the discussed studies on MSC implantation for IDD do not report significant osteophyte formation, this study illuminates an important potential safety consideration and has important clinical implications. Vadala et al. suggest that the use of a carrier gel or sealing agent may be able to prevent MSC extrusion from the disc and attenuate osteophyte formation. Finally, there exists a small risk of disease transmission in all cases of allograft implantation. As such, screening measures for transmissible diseases must be always employed prior to implantation.
Final Thoughts
Similar to gene therapy, the use of stem cells in the treatment of IDD holds much promise. Many questions regarding this therapy remain, however. One main concern is the optimal number of cells to inject in order to produce a desired effect is unknown. In addition, it is unknown whether the type of tissue from which the stem cells are obtained has an effect on the efficacy of transplantation. Furthermore, donor characteristics may influence the differentiation potential of the MSCs. Finally, long-term survivorship of the MSCs is unknown. Amongst the few studies that report this information a large degree of variability exists. One study reported a cellular attrition rate of 60% after four weeks31 while another reported no viable MSCs at twelve weeks34 whereas others have reported MSC viability up through 24 weeks,29 however this particular study did not quantify cell survival.
OXIDATIVE STRESS AND THE IVD
Background
The deleterious effects of reactive oxygen species (ROS) are well known, and it has been hypothesized that oxidative damage is involved in the cellular aging process though the specifics of this have yet to be fully elucidated. It has been shown that ROS in the form of hydrogen peroxide and superoxide radicals are produced in the IVD7,38 and are associated with significant negative effects on the disc environment. Hydrogen peroxide in the IVD has been shown to induce numerous signaling pathways resulting in senescent and catabolic changes in the NP cell consistent with the degenerative phenotype.5 Furthermore, disc cells cultured in supraphysiologic oxygen concentrations produce elevated levels of mitochondria-derived superoxide radicals which correlate with increased MMP levels and reduced proteoglycan content.38 These findings have raised new therapeutic possibilities for anti-oxidants in the treatment of IDD.
Therapeutic Agents
In the study, previously established to be a useful in vivo model to study IDD, Nasto et al. demonstrated that treatment of accelerated aging Ercc1(-/Δ) mice with the mitochondria-targeted ROS scavenger XJB-5-131 results in improved disc total glycosaminoglycan content and proteoglycan synthesis.38
Krupkova demonstrated that the polyphenol epigallocatechin gallate (EGCG) suppressed synthesis of senescence-associated β-galactosidase and increased the survival of human NP cells in vitro subjected to lethal doses of oxidative stress via hydrogen peroxide.39 They failed to show, however, that ECGC improved the proliferative capacity of the NP cells suggesting only partial attenuation of the oxidative effects.
Li et al. demonstrated that another phenolic anti-oxidant compound, piperine, produced many beneficial effects in the intervertebral disc of rats.40 In this study, lipopolysaccharide (LPS) was used to incite a potent inflammatory and catabolic response in the disc cells. Remarkably, piperine was able to counteract the LPS-induced upregulation in multiple oxidative-stress associated genes including interleukin-1 beta, tumor necrosis factor alpha, interleukin-6, nitric oxide, as well as numerous members of the matrix metalloproteinase (MMP) catabolic enzyme superfamily, such as MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5. Furthermore, piperine exhibited a protective effect on proteoglycan and type 2 collagen synthesis that was also reduced by LPS.
Yang et al. evaluated the in vitro effects of the endogenous antioxidant peptide glutathione on human NP cells exposed to either hydrogen peroxide or IL-1ß41. In the absence of glutathione, hydrogen peroxide exposed cells demonstrated dose-dependent apoptosis, decreased aggrecan and decreased type 2 collagen synthesis. Similarly, IL-1ß exposure reduced proteoglycan synthesis and increased nitric oxide levels. Glutathione administration significantly attenuated the effects of both hydrogen peroxide and IL-1ß. This study also supports the role of inflammatory cytokines in promoting oxidative pathways.
CONCLUSION
Currently, research regarding biologic approaches for IDD primarily focuses on gene and cell-based therapies though growing evidence supports a role for chemical agents that target oxidative stress and inflammation within the IVD. Data suggests these therapies are generally safe and have the potential to alter the degenerative course. However, the efficacy and safety of these therapies still require further investigation as does the long-term effects on pain and disability.
Finally, though sparse, data suggests that the degenerative disc cascade can be reversed or attenuated in its advanced stages with current biologic therapies. Besides the effect on pain and function, this is perhaps the most salient clinical question as the vast majority of patients with mild or early degenerative spine disease are asymptomatic and do not seek medical attention until the disease reaches its advanced stage. Current human clinical trials have demonstrated promising preliminary results and give cause for cautious optimism that biologic therapies have the potential to impact the disease course of IDD significantly.
SUGGESTED READINGS
- Nasto L, Seo H, Robinson A, et al. ISSLS prize winner: inhibition of NF-κB activity ameliorates age-associated disc degeneration in a mouse model of accelerated aging. Spine (Phila Pa 1976); 2012;37(21):1819-1825.
- Nishida K, Suzuki T, Kakutani K, et al. Gene therapy approach for disc degeneration and associated spinal disorders. Eur Spine J. 2008;17 Suppl 4:459-466.
- Vadala G, Russo F, Ambrosio L, Loppini M, Denaro V. Stem cells sources for intervertebral disc regeneration. World J Stem Cells. 2016;8(5):185-201.
REFERENCES
- Cassinelli EH, Hall RA, Kang JD. Biochemistry of intervertebral disc degeneration and the potential for gene therapy applications. Spine J. 2001;1(3):205-214.
- Trout JJ, Buckwalter JA, Moore KC. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat Rec. 1982;204(4):307-314.
- Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204(1):47-54.
- Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7(4):R732-745.
- Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater. 2015;30:89-102; discussion 103.
- Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T. A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int. 2016;2016:5952165.
- Kim KW, Chung HN, Ha KY, Lee JS, Kim YY. Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J. 2009;9(8):658-666.
- Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9(3):R45.
- Shimer AL, Chadderdon RC, Gilbertson LG, Kang JD. Gene therapy approaches for intervertebral disc degeneration. Spine (Phila Pa 1976). 2004;29(23):2770-2778.
- Vadala G, Sowa GA, Kang JD. Gene therapy for disc degeneration. Expert Opin Biol Ther. 2007;7(2):185-196.
- Nishida K, Doita M, Takada T, et al. Sustained transgene expression in intervertebral disc cells in vivo mediated by microbubble-enhanced ultrasound gene therapy. Spine (Phila Pa 1976). 2006;31(13):1415-1419.
- Chadderdon RC, Shimer AL, Gilbertson LG, Kang JD. Advances in gene therapy for intervertebral disc degeneration. Spine J. 2004;4(6 Suppl):341s-347s.
- Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J Bone Joint Surg Am. 1995;77(7):1103-1114.
- Lattermann C, Oxner WM, Xiao X, et al. The adeno associated viral vector as a strategy for intradiscal gene transfer in immune competent and pre-exposed rabbits. Spine (Phila Pa 1976). 2005;30(5):497-504.
- Moon SH, Gilbertson LG, Nishida K, et al. Human intervertebral disc cells are genetically modifiable by adenovirus-mediated gene transfer: implications for the clinical management of intervertebral disc disorders. Spine (Phila Pa 1976). 2000;25(20):2573-2579.
- Nishida K, Kang JD, Gilbertson LG, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine (Phila Pa 1976). 1999;24(23):2419-2425.
- Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine (Phila Pa 1976). 2004;29(23):2603-2611.
- Leckie SK, Bechara BP, Hartman RA, et al. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012;12(1):7-20.
- Paul R, Haydon RC, Cheng H, et al. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine (Phila Pa 1976). 2003;28(8):755-763.
- Levicoff EA, Kim JS, Sobajima S, et al. Safety assessment of intradiscal gene therapy II: effect of dosing and vector choice. Spine (Phila Pa 1976). 2008;33(14):1509-1516; discussion 1517.
- Afione SA, Wang J, Walsh S, Guggino WB, Flotte TR. Delayed expression of adeno-associated virus vector DNA. Intervirology. 1999;42(4):213-220.
- Vadala G, Sowa GA, Smith L, et al. Regulation of transgene expression using an inducible system for improved safety of intervertebral disc gene therapy. Spine (Phila Pa 1976). 2007;32(13):1381-1387.
- Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000;18(6):988-997.
- Gruber HE, Johnson TL, Leslie K, et al. Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine (Phila Pa 1976). 2002;27(15):1626-1633.
- Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Relat Res. 2001(389):94-101.
- Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23(3):403-411.
- Richardson SM, Hughes N, Hunt JA, Freemont AJ, Hoyland JA. Human mesenchymal stem cell differentiation to NP-like cells in chitosan-glycerophosphate hydrogels. Biomaterials. 2008;29(1):85-93.
- Strassburg S, Richardson SM, Freemont AJ, Hoyland JA. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med. 2010;5(5):701-711.
- Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8(6):888-896.
- Vadala G, Sobajima S, Lee JY, et al. In vitro interaction between muscle-derived stem cells and nucleus pulposus cells. Spine J. 2008;8(5):804-809.
- Anderson DG, Markova D, An HS, et al. Human umbilical cord blood-derived mesenchymal stem cells in the cultured rabbit intervertebral disc: a novel cell source for disc repair. Am J Phys Med Rehabil. 2013;92(5):420-429.
- Sakai D, Mochida J, Iwashina T, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27(3):335-345.
- Leckie SK, Sowa GA, Bechara BP, et al. Injection of human umbilical tissue-derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J. 2013;13(3):263-272.
- Ahn J, Park EM, Kim BJ, et al. Transplantation of human Wharton's jelly-derived mesenchymal stem cells highly expressing TGFβ receptors in a rabbit model of disc degeneration. Stem Cell Res Ther. 2015;6:190.
- Ltd. M. MPC-06-ID Phase 2 Chronic Low Back Pain Due to Disc Degeneration Clinical Trial. 2017; http://www.mesoblast.com/clinical-trial-results/mpc-06-id-phase-2.
- Coric D, Pettine K, Sumich A, Boltes MO. Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 Joint Spine Section Meeting. J Neurosurg Spine. 2013;18(1):85-95.
- Vadala G, Sowa G, Hubert M, Gilbertson LG, Denaro V, Kang JD. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6(5):348-355.
- Nasto LA, Robinson AR, Ngo K, et al. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. J Orthop Res. 2013;31(7):1150-1157.
- Krupkova O, Handa J, Hlavna M, et al. The natural polyphenol epigallocatechin gallate protects intervertebral disc cells from oxidative stress. Oxid Med Cell Longev. 2016;2016:7031397.
- Li Y, Li K, Hu Y, Xu B, Zhao J. Piperine mediates LPS induced inflammatory and catabolic effects in rat intervertebral disc. Int J Clin Exp Pathol. 2015;8(6):6203-6213.
- Yang D, Wang D, Shimer A, Shen FH, Li X, Yang X. Glutathione protects human nucleus pulposus cells from cell apoptosis and inhibition of matrix synthesis. Connect Tissue Res. 2014;55(2):132-139.