Ana V. Chee, Chundo Oh, Di Chen, Howard S. An
INTRODUCTION
The disc is composed of a gelatinous-like core tissue, nucleus pulposus (NP), surrounded by a tough outer tissue, annulus fibrosus (AF). The unique consistency of the NP is mainly due to its components—hydrophilic proteoglycan molecules and a loose network of collagens, mainly collagen type II. The AF is made up of highly organized collagen type I bundles which give it its tensile stiffness and strength. The function of the intervertebral disc is to maintain its structure and composition as it bears the loads and stresses of the spine. To maintain disc homeostasis, cells in the NP and AF tissues generate extracellular matrix proteins to replace normally degraded or damaged proteoglycan or collagen proteins. By studying NP and AF cells in vitro, different molecules such as growth factors and inflammatory cytokines can cause an imbalance in catabolic and anabolic processes. This chapter will review the initial in vitro studies of different growth factors, anti-inflammatory molecules and other biologics on NP and AF cells and their development as therapeutic agents on degenerated or painful intervertebral discs in animal models and clinical studies.
GROWTH FACTORS
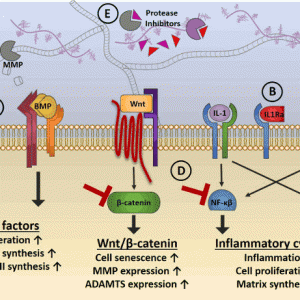
Growth factors bind to receptors on the cell surface to stimulate a variety of processes: growth, proliferation, differentiation or expression of genes important for tissue maintenance and repair (Fig. 5-1A). Using cultured canine intervertebral disc tissues, Thompson et al. were the first to show that growth factors could increase disc cell proliferation and proteoglycan synthesis.1 In their studies, NP tissues were more responsive to transforming growth factor-beta (TGF-β) and epidermal growth factor (EGF) than the other growth factors tested, fibroblast growth factor (FGF) and insulin-like growth factor-1 (IGF-1). Since this report, a plethora of in vitro studies have investigated the effects of a wide variety of growth factors on disc cells grown in monolayers, three-dimensional cultures or organ culture systems. Receptors for these growth factors (TGFβRII, BMPRII, FGFR3 and IGFRI) have been detected in non-degenerated and degenerated human disc tissues suggesting that disc tissues from different grades of degeneration should be responsive.2
TGF-β
To test the effects of TGF-β1 on human disc cells, Gruber et al. isolated AF cells from patients and donors of different age groups and cultured them in three-dimensional alginate layers.3 All samples showed an increase in cell proliferation on day 4 of culture and a decrease thereafter, while changes in expression of proteoglycan genes differed between samples when stimulated with TGF-β1.3 Risbud et al. tested isoforms TGF-β1 and TGF-β3 on whole rat disc organ cultures and found TGF-β3 was more effective in promoting disc cell phenotype and matrix production.4 These studies and many others have found that TGF-β treatment can yield controversial results depending on cell type, culture conditions, isoform, dosage and length of treatment. To circumvent these complications, novel strategies of combining TGF-β with other growth factors, cells or peptides that favor anabolic pathways are being explored.5-7 Combining TGF-β1 (1 ng/mL) with platelet-rich plasma (PRP) increased human NP cell proliferation rates in vitro.8 In an in vivo study, injecting rabbit injured discs with a mixture of TGF-β1, mesenchymal stem cells and fibrin glue was more advantageous than TGF-β1 and fibrin glue alone in reducing disc degeneration and apoptosis.6,8 Using a peptide that regulated the TGF-β1 pathway that favored anabolism, Kwon et al. found that injecting this peptide into the rabbit degenerated disc improved MRI and histological scores and increased proteoglycan contents.7 When comparing different growth factors in degenerated murine caudal discs in vivo, multiple injections of TGF-β1 had similar stimulatory effects on cell proliferation as a single injection of growth and differentiation factor 5 (GDF5).9 This may suggest that other growth factors have more potent chondrogenic effects than TGF-β and are worth exploring.
BONE MORPHOGENETIC PROTEINS
Bone morphogenetic proteins (BMPs) play important biological roles in embryonic development and tissue maintenance. Upon binding to their receptors, they regulate cell proliferation, differentiation and cell death. Although the majority of BMPs are involved in osteoblast differentiation and bone formation, they also contribute to the development of other tissues. BMP2, BMP7 (also known as osteogenic protein-1, OP-1) and BMP14 (also known as GDF5) have been shown to induce cartilage formation.10-13 Early studies showed that subcutaneous delivery of either BMP2 or BMP7 into the rat can induce ectopic bone and cartilage formation in 5 to 21 days.11,12,14 Later studies found that GDF5 stimulated a chondrogenic, not osteogenic, phenotype in vitro and in vivo.10,13,15
BMP7/OP-1
BMPs were tested to determine their potential effects on disc cell proliferation, extracellular matrix synthesis and tissue regeneration in vitro and in vivo. Recombinant human OP-1 (rhOP-1) was first tested on NP and AF cells isolated from rabbit intervertebral discs that were cultured in three-dimensional alginate beads.16 Rabbit NP and AF cells cultured in the presence compared to the absence of rhOP-1 had proliferated significantly after 7 days of culture. These cells also produced and accumulated significantly higher amounts of proteoglycan and collagen type II extracellular matrix proteins after 14 days of culture than the control counterparts that were cultured without rhOP-1.16 Later studies confirmed that rhOP-1 had similar effects on human NP and AF cells—inducing cell proliferation and proteoglycan synthesis and accumulation in vitro.17 The first studies to test growth factor intradiscal therapy in vivo were performed using rhOP-1 in the rabbit model.18-20 In non-degenerated rabbit discs, rhOP-1 injection caused significant increases in disc heights and NP proteoglycan contents at 2 weeks.18 Although proteoglycan increases were not sustained at later time points, the disc height increases were sustained at the 4- and 8-week time points.18 rhOP-1 demonstrated more drastic effects when the rabbit discs were degenerated.19,20 In these studies, rabbit disc degeneration was induced using annular puncture with an 18 gauge needle. Four weeks after injury when disc degeneration was significant, intradiscal rhOP-1 or lactose control treatments were administered. Treatment with rhOP-1 resulted in significantly better outcomes in MRI grading, biochemical analysis and histological scoring than lactose control-treated degenerated discs and restored disc heights and biomechanical properties to near non-degenerated disc levels.19,20
BMP2
In vitro studies testing recombinant human BMP2 (rhBMP2) on human and rat disc cells also showed proliferative effects and stimulation of chondrogenic genes.21-23 Using the rat tail model, rhBMP2 injection increased disc height temporarily for 2 weeks.24 Thereafter, the disc heights returned to degenerated levels.24 Simvastatin, a statin that can upregulate BMP2 and promote chondrogenesis, was injected into degenerated discs of rats.25 Low doses of simvastatin delivered with a hydrogel showed improved MRI indices, histological grades and expression of aggrecan and collagen type II compared to hydrogel alone or high doses of simvastatin.25 When tested in the rabbit annular stab model, treatment with rhBMP2 unexpectedly gave rise to increased vascularity, fibroblast proliferation and degeneration.26 These animal studies suggest that BMP2 dosage and time of treatment after disc injury may affect the outcome.
GDF5
GDF5 was also tested for its therapeutic potential on disc cells and tissues in vitro and in vivo.9,27-30 Using bovine NP and AF cells cultured in 3-D alginate beads, recombinant human GDF5 (rhGDF5) promoted cell proliferation and extracellular matrix protein synthesis.27 In both human and mouse cultured disc cells, aggrecan and collagen type II gene expression increased in the presence of GDF5.28,29 In the rabbit disc degeneration model, intradiscal injection of GDF5 resulted in increases in disc height and improvements in MRI grades and histological scores.27 Lastly, when microsphere-encapsulated rhGDF5 was delivered into rat degenerated caudal discs, there were improvements in disc height indexes, proteoglycan contents and gene expression of collagen type II.30
Both Stryker Corporation and Depuy Spine, Inc. started FDA approved Phase 1 trial on the safety of intradiscal injection of OP-1 and GDF-5, respectively. The Phase 2 studies have not begun on these growth factors.
PRP
Platelet-rich plasma (PRP) is a plasma fraction prepared from autologous blood containing a higher than baseline platelet concentration. Platelets store an admixture of bioactive proteins and growth factors that can help with wound healing and tissue regeneration. These factors include TGF-β, platelet-derived growth factor (PDGF), IGF-1, FGF, EGF, vascular endothelial growth factor (VEGF), and endothelial cell growth factor (ECGF). Before PRP can be used as a therapeutic agent, platelets are activated with thrombin, calcium chloride, or collagen type I allowing the growth factors to be released. Due to the autologous nature of this product, ease of preparation and variable successes, PRP treatment has gained popularity in the regenerative fields of dermatology, plastic surgery, orthopaedics and sports medicine. In vitro and in vivo studies using PRP to treat degenerative disc models have had promising results. In vitro studies showed that PRP has both stimulatory and anti-inflammatory effects on disc cells.31,32 Using porcine NP and AF cells grown in alginate beads, PRP mildly stimulated cell proliferation and significantly increased proteoglycan and collagen synthesis.31 Using porcine AF cells grown in monolayer, the presence of PRP was able to counter the effects of TNFα treatment by upregulating aggrecan and collagen types I and II genes and downregulating matrix metalloproteinase genes.32 These studies demonstrated the potential therapeutic effects of PRP.
The results of PRP treatment in vivo have been variable mainly due to the variation in animal models and different protocols for preparation and activation of PRP. Using the rat injury model, Gullung et al. found that PRP treatment reduced disc degeneration and immune cell infiltration into injured discs.33 Using the porcine model, treatment with PRP activated with thrombin caused an increase in collagen type II and aggrecan gene expression in degenerated discs yet did not significantly increase disc height.34 Degenerated rabbit discs treated with un-activated PRP or un-activated PRP followed by injection of activating agents (thrombin and CaCl2) did not show significant increases in disc height, MRI grading or expression of matrix proteins.35,36 Suspecting that activator thrombin may cause some catabolic activities in the disc, Obata et al. decided to activate PRP using CaCl2 and autologous rabbit serum, centrifuge out the insoluable fraction, and save the soluable fraction (PRP-releasate) for treatment.37 When PRP-releasate was injected into rabbit degenerated discs, there were significant increases in disc height indexes and the number of chondrocyte-like cells in the NP and anterior AF.37 Several studies that combined PRP with MSCs for treatment also gave mixed results. Chen et al. found that treatment with PRP and MSCs on porcine degenerated discs promoted a more osteogenic phenotype rather than a chondrogenic one,34 while Hou et al. found this combination treatment did increase proteoglycan and collagen type II contents in rabbit degenerated discs.36 These studies suggest that more work will be needed to optimize the preparation and activation of PRP for disc treatment.
Two clinical trials testing intradiscal injection of autologous PRP alone or as part of a combination treatment for back pain have been published.38,39 The first study was a prospective, double-blind, randomized controlled trial that compared autologous PRP treatment with contrast control treatment in 49 patients.38 After 8 weeks of treatment, the investigators found that patients treated with PRP had significant improvements in the measures for Functional Rating Index (FRI), Numeric Rating Scale (NRS) for pain and patient satisfaction using the North American Spine Society (NASS) Outcome Questionnaire. Since the control patients did not see improvements at 8 weeks, they were then offered the PRP treatment after this discovery and were no longer eligible as controls for the remaining of the study. At the 1 year follow up, improvements persisted in the PRP treatment group as average pain scores were significantly better than their baseline.38 In another study, the safety and efficacy of combining autologous PRP and autologous stromal vascular fraction (SVF) as a treatment for back pain was tested in 15 patients.39 The average changes in pain ratings, visual analog scores and present pain indexes significantly improved after 6 months of combination treatment compared to baseline. No adverse effects were reported 12 months after treatment.39 Although both PRP clinical trials showed promising results, more studies may be needed to determine candidate patient population and to standardize protocols for the preparation, activation and dosage of PRP intradiscal treatment.
TARGETING INFLAMMATORY PATHWAYS
Inflammatory cytokines are associated with disc herniation, disc degeneration and discogenic pain. Initiation of inflammation may be due to structural damage, improper loading, loss of homeostasis or excessive levels of extracellular matrix degraded products. Studies on surgical samples removed from patients with disc herniation, degenerated disc disease or spondylosis show immunopositive staining for TNFα and IL1β.40-42 Receptors for TNFα and IL1β have also been detected in disc cells.42,43 These cytokines appear to be the key regulators in inflammation initiated by disc cells. In vitro studies show that cultured disc cells respond to TNFα and IL1β by upregulating expression of catabolic genes—pro-inflammatory cytokines (e.g. IL-6 and IL-8), matrix metalloproteases and chemokines. Expression and synthesis of pro-inflammatory cytokines, IL-6 and IL-8, have also been detected in surgical disc tissues or explant cultures of these tissues.44-49 Several chemokines, C-C chemokine ligand (CCL)2, CCL3 and CCL5, have been found to be expressed by disc cells and detected in painful discs.48,50-53 Chemokines released by degenerated or diseased discs can attract macrophages and other immune cells to help repair the tissue. In normal conditions, resolution occurs after the tissue has healed. If the problem persists and resolution does not occur, immune cells present in the disc can continue to increase inflammation, degeneration and disease. Targeting inflammatory pathways may slow down the cascade of events that eventually lead to discogenic pain.
INTERLEUKIN-1 RECEPTOR ANTAGONIST
Interleukin-1 receptor antagonist (IL-1Ra) is a naturally occurring protein that binds to interleukin-1 receptor (IL-1R) with high affinity without activating a cellular response (Fig. 5-1B). Although presence of IL-1β, IL-1α and receptor immunopositive cells increases in disc tissues of advancing degrees of degeneration, IL-1Ra expression appears to remain stable in both non-degenerated and degenerated discs.43 In vitro studies of rabbit, bovine and human disc cells have found that the presence of recombinant human IL-1Ra (rhIL-1Ra) can prevent downstream inflammatory events.54-56 Maeda et al. found that rabbit inner and outer AF cells cultured in the presence of rhIL-1Ra prevented the inhibitory effects of IL-1α on proteoglycan synthesis.54 Using in situ zymography, Le Matire et al. found that collagenase, gelatinase and caseinase activities decreased in moderately degenerated human disc explant tissues when incubated with IL-1Ra.56 Lastly, IL-1Ra was able to counter the effects of IL-1β treatment in bovine disc cells cultured in alginate by restoring proteoglycan contents, biomechanical properties and gene expression of matrix proteins and proteinases to control levels.55
In order for IL-1Ra therapy to be effective, high concentrations of this protein would be needed to compete out endogenous IL-1α and IL-1β and to overcome other natural regulators. Several strategies have been designed and tested to circumvent the need for a treatment of repeat doses of high concentrations of the drug. One strategy was to deliver IL-1Ra through a cell-based gene therapy approach. Disc cells transfected with an adenovirus vector expressing IL-1Ra was injected into moderately degenerated disc explant tissues and found to be effective in reducing matrix metalloproteinase (MMP) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) expression for up to 14 days.56 Another approach utilized a recombinant fusion protein composed of the bioactive domains of IL-1Ra and an elastin-like polypeptide (ELP) that aggregates at physiological temperatures and slowly gets released into the microenvironment.57 When tested on human NP and AF cells cultured in alginate beads, the ELP-IL-1Ra fusion protein was able to prevent IL-1 induced expression of MMP3, ADAMTS4 and TNFα.57 Lastly, another method of sustained delivery of IL-1Ra was developed by generating poly(lactic-co-glycolic acid) (PLGA) microspheres combined with IL-1Ra.58 Seven days after IL-1Ra microspheres were co-injected with IL-1β into rat caudal discs, the catabolic effects of IL-1β were inhibited and proteoglycan contents were similar to those of untreated intact discs.59 Although these IL-1Ra delivery strategies appear to be effective in these model systems, the testing of these molecules in larger animal studies and for longer periods of time remains to be determined.
TUMOR NECROSIS FACTOR-α INHIBITORS
Targeting tumor necrosis factor-α (TNF-α) pathway has been effective in treating various inflammatory diseases such as rheumatoid arthritis, Crohn’s disease and psoriasis. Inhibitors include antibodies and soluble receptors that bind and neutralize soluble TNF-α, resulting in an overall decrease in production of pro-inflammatory cytokines and infiltration of immune cells (Fig. 5-1C). Currently, there are only a few published studies that have tested the effects of TNF-α inhibitors on disc cells in vitro and in vivo. Using human disc cells, Sinclair et al. found that soluble TNF receptor II (sTNFRII) treatment prevented TNF-α induced upregulation of nitric oxide and prostaglandin E2 while IL-6 levels remained unchanged. In an in vivo model, TNF-α inhibitor was injected into rat injured L4/5 disc and the number of innervating dorsal root ganglia (DRG) neurons expressing the pain marker, calcitonin gene-related peptide (CGRP), was analyzed.60,61 Both studies found that treatment with TNF-α inhibitor caused a significant decrease in pain marker expression in the DRGs compared to injured discs treated with saline.60,61 Several clinical studies have investigated the therapeutic effects of TNF-α inhibitors on patients with sciatica and discogenic back pain.
Multiple double-blinded, placebo-controlled clinical trials testing anti-TNF-α antibodies (infliximab or adalimumab) or TNF-α decoy receptor (etanercept) had mixed results on patients with sciatica and discogenic pain. The trials varied in outcome measures, delivery and concentration of the drugs, patient selection and follow-up terms. In the first trial, intravenous delivery of infliximab in patients with sciatica showed similar results to placebo at both 3-month and 1-year follow up time points.62,63 Two clinical studies using epidural injections of etanercept found significant improvements compared to control treatments in leg and back pain scores in sciatica patients at different time points post-treatment, from 2 to 3 weeks or from 1 to 6 months.64,65 Lastly, a longer-term clinical study that treated sciatica patients with two subcutaneous injections of adalimumab at 7-day intervals reported a significant decrease in leg and back pain after 6 months and a decrease in surgeries performed on these patients after 3 years.66,67 Intradiscal injection of etanercept into patients with discogenic back pain also reported mixed results.68,69 Patients treated with low doses of etanercept, 0.1 to 1.5 mg, showed no differences in pain or disability scores compared to those treated with saline after 1 month.68 In the other study where patients were treated with higher amounts of etanercept (10 mg), there was a significant difference in pain scores at 4 and 8 weeks after treatment and disability scores only after 4 weeks after treatment.69
OTHER INFLAMMATORY TARGETS
IL-6 and IL-8
Inflammatory cytokines, IL-6 and IL-8, have been shown to be upregulated in disc tissues obtained from patients undergoing back surgery or disc cells cultured from these patients.44-47,49,51,70 Degenerated discs of rabbits, minipigs and mice or herniated disc material of rats have been reported to express higher levels of IL-6 or IL-8.71-76 These inflammatory cytokines are potential targets to prevent disc inflammation and back pain. In the rat disc herniation model, intrathecal administration of small molecule IL-8 receptor inhibitor near dorsal root ganglia reduced pain behavior and activation of microglia cells of the spinal dorsal horn.72 Treatment with anti-IL-6 receptor antibodies in murine injured discs reduced the number of dorsal root ganglia neurons expressing pain-related peptide, CGRP.75 A clinical study reported that treatment with IL-6 receptor antibody tocilizumab on patients with discogenic back pain had temporary improvements in numeric rating scale pain scores and Oswestry disability index scores.77
NF-κB and p38 MAPK
Signaling molecules that play important roles in translating the inflammatory response can also serve as therapeutic targets. NF-κB and p38 MAPK are important signaling molecules that are activated by IL-1β or TNFα and are found to be activated in aging intervertebral discs (Fig. 5-1D).78-81 In an accelerated aging mouse model, inhibiting activation of NF-κB in aging discs reduced disc degeneration and increased proteoglycan synthesis.81 In vitro studies showed that NF-κB or p38 MAPK inhibitors reduced catabolic and inflammatory gene expression induced by IL-1β or TNFα in disc cells.78-80,82,83 Compared to saline treated injured discs, rat injured discs treated with small-molecule IκB kinase-β (IKKβ) inhibitor, ultimately preventing NF-κB activation, had reduced IL-1β, TNFα and IL-6 production.84 The IKKβ inhibitor treated group also showed the potential for reduced pain given that the percentage of CGRP positive infiltrating dorsal root ganglia neurons expressing CGRP was significantly lower.84
Chemokines and Chemokine Receptors
Targeting chemokines or chemokine receptors may be a promising treatment strategy for diseases with chronic inflammation such as rheumatoid arthritis (RA) and discogenic back pain. Infiltration of macrophages and neutrophils into peripheral tissues is an important mechanism to remove tissues that have been damaged or infected. In diseases with chronic inflammation such as RA, immune cells can cause structural damage and morbidity to the joints. To decrease immune cell migration and further destruction of tissues, chemokines (CCL2, CCL3 and CCL5) and chemokine receptors [C-C chemokine receptor (CCR1, CCR2 and CCR5)] have been targeted for inhibition. Neutralizing antibodies or small molecule antagonists against these targets and others have been tested in inhibiting macrophage migration in vitro and preventing disease pathogenesis in vivo with a range of results from not effective to successful.11 After decades of clinical trials with different inhibitors, a small molecule antagonist against CCR1 was found to be safe, tolerable and clinically active in reducing inflammation in RA patients.12-15 Chemokines are known to be expressed and released by disc cells48,50-53 which may serve to recruit macrophages detected in degenerated or painful discs.85,86 Several in vitro studies have reported that small molecule antagonists against CCR150,87 and CCR253 can inhibit macrophage migration induced by disc cells. In a rabbit study, rabbit discs were injured and treated with saline or antagonists against CCR1 or CCR2. Discs treated with CCR1 inhibitor had better MRI grades, a higher ratio of collagen type 2 to collagen type 1 gene expression, and lesser inflammatory marker expression than discs treated with saline or CCR2 antagonists.87 In the long-term, intradiscal injection of either of these antagonists did not block macrophage migration into the rabbit discs suggesting that delivery of these antagonists may need to be in multiple doses or continuous.
OTHER TARGETS
Wnt/β-catenin
The Wnt family of proteins influences a spectrum of biological processes, including initial embryonic development, tissue organization, self-renewal, cell proliferation and differentiation, and pathological processes such as tumorigenesis. Wnt/β-catenin signaling, also known as canonical Wnt signaling, is dependent on sequential molecular events involving β-catenin. To investigate the role of Wnt/β-catenin signaling in regulation of IVD development and organization, several studies have been elucidated. Hiyama et al. demonstrated that Wnt/β-catenin signaling accelerates the senescence of NP cells and induces the expression of MMPs that are involved in the breakdown of extracellular matrix and the progression of disc disease using in vitro studies in rat IVD cells.88 In an in vivo study by Kondo et al., temporal-spatial distribution of Wnt/β-catenin signaling activity was examined in IVD using Wnt/ β-catenin reporter (TOPGAL) mice. At an embryonic stage, the signaling activities were found in the AF as well as cartilaginous elements such as the growth plate and the endplate, but lower in the NP. However the signaling activity weakened with maturation of the IVD structure, but appeared in NP cells at older age suggesting that proper control of this signaling would be critical to establish IVD structure and to support maturation of IVD organization.89 In the study of the significance of Wnt/β-catenin signaling during IVD organization, Kondo et al. found conditional deletion of β-catenin in Col2a1-expressing cells leads to increased endochondral bone formation in the end plate.89 Since β-catenin protein was upregulated in disc tissues from patients with disc degeneration, the effect of increased β-catenin on disc tissue was examined using β-catenin conditional activation mice.90 In these mice, severe defects in disc tissue were found including upregulation of expression of Mmp13 and Adamts4 and Adamts5 genes, significant loss of growth plate cartilage and severe osteophyte formation.90 These findings demonstrate that Wnt/β-catenin signaling plays a critical role in disc tissue function and may be involved in the development of disc degeneration (Fig. 5-1D).
Reactive Oxygen Species
Oxidative stress can occur in disc tissues when reactive oxygen species (ROS) are generated and not removed. This accumulation may occur due to the natural aging process or exogenous stressors. Nitric oxide (NO) is a ROS that has been known to be generated by disc cells from herniated tissues or after treatment with inflammatory cytokines or mechanical stretching.44,79,91,92 High levels of ROS exposure can induce cell senescence, apoptosis and expression of inflammatory cytokines and matrix-degrading metalloproteinases in disc cells.93-96 Antioxidant treatment with nanofullerol or N-acetyl cysteine (NAC) reversed these cell phenotypes.92-94 Antioxidant treatment in several animal models was effective in reversing disc degeneration. In the rat tail injury model, continuous oral administration of antioxidant N-acetyl cysteine reduced disc degeneration.94 In the rabbit disc injury model, intradiscal injection of nanofullerol prevented loss of proteoglycan.92
Resveratrol is a natural anti-oxidant found in grapes and red wines and known to exhibit cardioprotective and anti-inflammatory effects. In vitro studies have demonstrated that resveratrol can prevent IL-1 induced upregulation of matrix-degrading enzymes and inflammatory cytokines in bovine and human disc cells.97,98 In vivo, resveratrol can improve MRI grades and increase aggrecan gene expression in the rabbit disc degeneration model and reduce NP-mediated pain behavior in rat model of radiculopathic pain.98,99 Other natural compounds that have been reported to reduce inflammation and catabolic activities in disc cells are vitamin D and lactoferricin.100,101
Matrix-degrading Enzymes
Two families of matrix-degrading enzymes, MMPs and ADAMTSs, have been well studied in facilitating the progression of disc degeneration. Aging, mechanical stress and inflammation can trigger the upregulation of these genes in disc cells. In human disc samples, Le Maitre et al. observed that the numbers of cells expressing MMP1, 3, 7 and 13 and ADAMTS4 increased as the degree of degeneration advanced.102,103 In vitro studies showed that treating NP or AF cells with inflammatory cytokines, IL-1β or TNFα, increased gene expression of MMP1, 3 and 13 ADAMTS4 and ADAMTS5.104-106 Several in vivo studies confirmed that the genes of these MMPs and ADAMTSs were also upregulated in rabbit discs after injury.107-109 Tissue inhibitors of metalloproteinases (TIMPs) are proteins that can modulate the activity of MMPs. Immunopositivity of TIMP1 and TIMP2 correlated with the expression of MMPs in human disc tissue samples.102 In the rabbit model, delivery of TIMP1 gene through an adeno-associated virus vector into rabbit injured discs delayed disc degeneration.110 These studies suggest that inhibiting MMPs may reduce disc degeneration (Fig. 5-1E). Several osteoarthritis clinical trials with MMP inhibitors have reported mixed results. One trial observed that treatment with doxycycline, an antibiotic that also inhibits MMP activity, reduced radiographic progression of disease but did not change joint pain, while the other trial found the treatment with a broad spectrum MMP inhibitor was not effective in knee osteoarthritis and resulted in some musculoskeletal side effects.111,112
CONCLUSIONS
Tissue culture studies with disc cells have been valuable in enriching our understanding of molecular mechanisms of disc degeneration. Animal studies of disc degeneration have enabled designing and testing of novel therapeutics for disc regeneration and pain reduction. Targeting the inhibition of inflammatory cytokines or enhancing cell proliferation with growth factors have proven to alter disc homeostasis in favor of regeneration in vitro and in vivo. As these biological therapies are translated into clinical trials, the outcomes indicate that symptomatic discs of patients may be more complicated to treat than those in our current model systems. Since biological molecules have short half-lives, beneficial effects of a single dose treatment may be limited. Also, as exemplified by the success of PRP studies, treatment designed with one molecule may not cover multiple disease pathways seen in patients. Therefore, effective treatment strategies may need to incorporate repeat dosing or a system of continuous, gradual release of therapeutics and an admixture of multiple growth factors and anti-inflammatory agents. In the age of personalized medicine, understanding the microenvironment of an individual’s diseased tissue will allow us to design a tailor-made cocktail of anti-inflammatories and growth factors to treat disc diseases and pain.
REFERENCES
- Thompson JP, Oegema TR Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine (Phila Pa 1976). 1991;16(3):253-260.
- Le Maitre CL, Richardson SM, Baird P, Freemont AJ, Hoyland JA. Expression of receptors for putative anabolic growth factors in human intervertebral disc: implications for repair and regeneration of the disc. J Pathol. 2005 Dec;207(4):445-452.
- Gruber HE, Fisher EC,Jr., Desai B, Stasky AA, Hoelscher G, Hanley EN Jr. Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235(1):13-21.
- Risbud MV, Di Martino A, Guttapalli A, et al. Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine (Phila Pa 1976). 2006;31(8):884-890.
- Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15 Suppl 3:303-311.
- Yang H, Wu J, Liu J, et al. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J. 2010;10(9):802-810.
- Kwon YJ, Lee JW, Moon EJ, Chung YG, Kim OS, Kim HJ. Anabolic effects of Peniel 2000, a peptide that regulates TGF-β1 signaling on intervertebral disc degeneration. Spine (Phila Pa 1976). 2013;38(2):E49-58.
- Chen WH, Lo WC, Lee JJ, et al. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006;209(3):744-754.
- Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29(2):156-163.
- Hotten GC, Matsumoto T, Kimura M, et al. Recombinant human growth/differentiation factor 5 stimulates mesenchyme aggregation and chondrogenesis responsible for the skeletal development of limbs. Growth Factors. 1996;13(1-2):65-74.
- Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528-1534.
- Sampath TK, Maliakal JC, Hauschka PV, et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267(28):20352-20362.
- Erlacher L, McCartney J, Piek E, et al. Cartilage-derived morphogenetic proteins and osteogenic protein-1 differentially regulate osteogenesis. J Bone Miner Res. 1998 Mar;13(3):383-392.
- Wang EA, Rosen V, D'Alessandro JS, et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2220-2224.
- Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100(2):321-330.
- Masuda K, Takegami K, An H, et al. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21(5):922-930.
- Imai Y, Miyamoto K, An HS, Thonar EJ, Andersson GB, Masuda K. Recombinant human osteogenic protein-1 upregulates proteoglycan metabolism of human anulus fibrosus and nucleus pulposus cells. Spine. 2007;32(12):1303-1309; discussion 1310.
- An HS, Takegami K, Kamada H, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine (Phila Pa 1976). 2005;30(1):25-31; discussion 31-32.
- Masuda K, Imai Y, Okuma M, et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine (Phila Pa 1976). 2006;31(7):742-754.
- Miyamoto K, Masuda K, Kim JG, et al. Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 2006;6(6):692-703.
- Tim Yoon S, Su Kim K, Li J, et al. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine. 2003;28(16):1773-1780.
- Li J, Yoon ST, Hutton WC. Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J Spinal Disord Tech. 2004;17(5):423-8.
- Kim H, Lee JU, Moon SH, et al. Zonal responsiveness of the human intervertebral disc to bone morphogenetic protein-2. Spine (Phila Pa 1976). 2009;34(17):1834-1838.
- Inoue H, Montgomery SR, Aghdasi B, et al. The effect of bone morphogenetic protein-2 injection at different time points on intervertebral disk degeneration in a rat tail model. J Spinal Disord Tech. 2015;28(1):E35-44.
- Than KD, Rahman SU, Wang L, et al. Intradiscal injection of simvastatin results in radiologic, histologic, and genetic evidence of disc regeneration in a rat model of degenerative disc disease. Spine J. 2014;14(6):1017-1028.
- Huang KY, Yan JJ, Hsieh CC, Chang MS, Lin RM. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: an animal experiment. Spine (Phila Pa 1976). 2007;32(11):1174-1180.
- Chujo T, An HS, Akeda K, et al. Effects of growth differentiation factor-5 on the intervertebral disc--in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976). 2006;31(25):2909-2917.
- Cui M, Wan Y, Anderson DG, et al. Mouse growth and differentiation factor-5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. Spine J. 2008;8(2):287-295.
- Le Maitre CL, Freemont AJ, Hoyland JA. Expression of cartilage-derived morphogenetic protein in human intervertebral discs and its effect on matrix synthesis in degenerate human nucleus pulposus cells. Arthritis Res Ther. 2009;11(5):R137.
- Yan J, Yang S, Sun H, et al. Effects of releasing recombinant human growth and differentiation factor-5 from poly(lactic-co-glycolic acid) microspheres for repair of the rat degenerated intervertebral disc. J Biomater Appl. 2014;29(1):72-80.
- Akeda K, An HS, Pichika R, et al. Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine (Phila Pa 1976). 2006;31(9):959-966.
- Cho H, Holt DC 3rd, Smith R, Kim SJ, Gardocki RJ, Hasty KA. The effects of platelet-rich plasma on halting the progression in porcine intervertebral disc degeneration. Artif Organs. 2016;40(2):190-195.
- Gullung GB, Woodall JW, Tucci MA, James J, Black DA, McGuire RA. Platelet-rich plasma effects on degenerative disc disease: analysis of histology and imaging in an animal model. Evid Based Spine Care J. 2011;2(4):13-18.
- Chen WH, Liu HY, Lo WC, et al. Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials. 2009;30(29):5523-5533.
- Sawamura K, Ikeda T, Nagae M, et al. Characterization of in vivo effects of platelet-rich plasma and biodegradable gelatin hydrogel microspheres on degenerated intervertebral discs. Tissue Eng Part A. 2009;15(12):3719-3727.
- Hou Y, Shi G, Shi J, Xu G, Guo Y, Xu P. Study design: in vitro and in vivo assessment of bone morphogenic protein 2 combined with platelet-rich plasma on treatment of disc degeneration. Int Orthop. 2016;40(6):1143-1155.
- Obata S, Akeda K, Imanishi T, et al. Effect of autologous platelet-rich plasma-releasate on intervertebral disc degeneration in the rabbit anular puncture model: a preclinical study. Arthritis Res Ther. 2012;14(6):R241.
- Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, et al. Lumbar intradiskal platelet-rich plasma (PRP) injections: a prospective, double-blind, randomized controlled study. PM R. 2016;8(1):1-10; quiz 10.
- Kristin C, Robert S, Michelle P. Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J Transl Med. 2017 Jan 13;15(1):12.
- Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine (Phila Pa 1976). 2005;30(1):44-53; discussion 54.
- Kokubo Y, Uchida K, Kobayashi S, et al. Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J Neurosurg Spine. 2008;9(3):285-295.
- Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9(4):R77.
- Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7(4):R732-45.
- Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF 3rd, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976). 1996;21(3):271-277.
- Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine (Phila Pa 1976). 1996;21(2):218-224.
- Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84(2):196-201.
- Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine (Phila Pa 1976). 2002;27(9):911-917.
- Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Spontaneous production of monocyte chemoattractant protein-1 and interleukin-8 by the human lumbar intervertebral disc. Spine (Phila Pa 1976). 2002;27(13):1402-1407.
- Shamji MF, Setton LA, Jarvis W, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62(7):1974-1982.
- Wang J, Tian Y, Phillips KL, et al. Tumor necrosis factor α- and interleukin-1β-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 2013;65(3):832-842.
- Kepler CK, Markova DZ, Dibra F, et al. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL-1β in painful human intervertebral discs. Spine (Phila Pa 1976). 2013;38(11):873-880.
- Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Norton HJ, Hanley EN Jr. Production and expression of RANTES (CCL5) by human disc cells and modulation by IL-1-β and TNF-α in 3D culture. Exp Mol Pathol. 2014;96(2):133-138.
- Shi P, Chee AV, Liu DK, et al. Chemokine receptor antagonists can inhibit macrophage migration induced by annulus fibrosus and nucleus pulposus cells. International Society for the Advancement of Spine Surgery Annual Meeting; San Diego, CA; 2015.
- Maeda S, Kokubun S. Changes with age in proteoglycan synthesis in cells cultured in vitro from the inner and outer rabbit annulus fibrosus. Responses to interleukin-1 and interleukin-1 receptor antagonist protein. Spine (Phila Pa 1976). 2000;25(2):166-169.
- Smith LJ, Chiaro JA, Nerurkar NL, et al. Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine challenge following long-term agarose culture. Eur Cell Mater. 2011;22:291-301.
- Le Maitre CL, Hoyland JA, Freemont AJ. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis Res Ther. 2007;9(4):R83.
- Shamji MF, Betre H, Kraus VB, et al. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: sustained release of a local antiinflammatory therapeutic. Arthritis Rheum. 2007;56(11):3650-3661.
- Gorth DJ, Mauck RL, Chiaro JA, et al. IL-1ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1β-mediated degradation of nucleus pulposus in vitro. Arthritis Res Ther. 2012;14(4):R179.
- Gorth DJ, Martin JT, Dodge GR, et al. In vivo retention and bioactivity of IL-1ra microspheres in the rat intervertebral disc: a preliminary investigation. J Exp Orthop. 2014;1(1):15.
- Inage K, Orita S, Yamauchi K, et al. Dose optimization for single intradiscal administration of the tumor necrosis factor-α inhibitor, etanercept, in rat disc injury models. Asian Spine J. 2016;10(4):619-623.
- Horii M, Orita S, Nagata M, et al. Direct application of the tumor necrosis factor-α inhibitor, etanercept, into a punctured intervertebral disc decreases calcitonin gene-related peptide expression in rat dorsal root ganglion neurons. Spine (Phila Pa 1976). 2011;36(2):E80-85.
- Korhonen T, Karppinen J, Paimela L, et al. The treatment of disc herniation-induced sciatica with infliximab: results of a randomized, controlled, 3-month follow-up study. Spine (Phila Pa 1976). 2005;30(24):2724-2728.
- Korhonen T, Karppinen J, Paimela L, et al. The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine (Phila Pa 1976). 2006;31(24):2759-2766.
- Cohen SP, Bogduk N, Dragovich A, et al. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology. 2009;110(5):1116-1126.
- Ohtori S, Miyagi M, Eguchi Y, et al. Epidural administration of spinal nerves with the tumor necrosis factor-alpha inhibitor, etanercept, compared with dexamethasone for treatment of sciatica in patients with lumbar spinal stenosis: a prospective randomized study. Spine (Phila Pa 1976). 2012;37(6):439-444.
- Genevay S, Viatte S, Finckh A, Zufferey P, Balague F, Gabay C. Adalimumab in severe and acute sciatica: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(8):2339-2346.
- Genevay S, Finckh A, Zufferey P, Viatte S, Balague F, Gabay C. Adalimumab in acute sciatica reduces the long-term need for surgery: a 3-year follow-up of a randomised double-blind placebo-controlled trial. Ann Rheum Dis. 2012;71(4):560-562.
- Cohen SP, Wenzell D, Hurley RW, et al. A double-blind, placebo-controlled, dose-response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology. 2007;107(1):99-105.
- Sainoh T, Orita S, Miyagi M, et al. Single intradiscal administration of the tumor necrosis factor-alpha inhibitor, etanercept, for patients with discogenic low back pain. Pain Med. 2016;17(1):40-45.
- Zhang Y, Chee A, Shi P, et al. Intervertebral disc cells produce interleukins found in patients with back pain. Am J Phys Med Rehabil. 2016;95(6):407-415.
- O'Neill CW, Liu JJ, Leibenberg E, et al. Percutaneous plasma decompression alters cytokine expression in injured porcine intervertebral discs. Spine J. 2004;4(1):88-98.
- Kim SJ, Park SM, Cho YW, et al. Changes in expression of mRNA for interleukin-8 and effects of interleukin-8 receptor inhibitor in the spinal dorsal horn in a rat model of lumbar disc herniation. Spine (Phila Pa 1976). 2011;36(25):2139-2146.
- Takada T, Nishida K, Maeno K, et al. Intervertebral disc and macrophage interaction induces mechanical hyperalgesia and cytokine production in a herniated disc model in rats. Arthritis Rheum. 2012;64(8):2601-2610.
- Kuelling FA, Foley KT, Liu JJ, et al. The anabolic effect of plasma-mediated ablation on the intervertebral disc: stimulation of proteoglycan and interleukin-8 production. Spine J. 2014;14(10):2479-2487.
- Sainoh T, Orita S, Miyagi M, et al. Interleukin-6 and interleukin-6 receptor expression, localization, and involvement in pain-sensing neuron activation in a mouse intervertebral disc injury model. J Orthop Res. 2015;33(10):1508-1514.
- Zhang Y, Chee A, Shi P, et al. Allogeneic articular chondrocyte transplantation downregulates interleukin 8 gene expression in the degenerating rabbit intervertebral disk in vivo. Am J Phys Med Rehabil. 2015;94(7):530-538.
- Sainoh T, Orita S, Miyagi M, et al. Single intradiscal injection of the interleukin-6 receptor antibody tocilizumab provides short-term relief of discogenic low back pain; prospective comparative cohort study. J Orthop Sci. 2016;21(1):2-6.
- Studer RK, Aboka AM, Gilbertson LG, et al. p38 MAPK inhibition in nucleus pulposus cells: a potential target for treating intervertebral disc degeneration. Spine. 2007;32(25):2827-2833.
- Studer RK, Gilbertson LG, Georgescu H, Sowa G, Vo N, Kang JD. p38 MAPK inhibition modulates rabbit nucleus pulposus cell response to IL-1. J Orthop Res. 2008;26(7):991-998.
- Wang X, Wang H, Yang H, et al. Tumor necrosis factor-α- and interleukin-1β-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-NF-κB axis: implications in inflammatory disc disease. Am J Pathol. 2014;184(9):2560-2572.
- Nasto LA, Seo HY, Robinson AR, et al. ISSLS prize winner: inhibition of NF-κB activity ameliorates age-associated disc degeneration in a mouse model of accelerated aging. Spine (Phila Pa 1976). 2012;37(21):1819-1825.
- Tisherman R, Coelho P, Phillibert D, et al. NF-κB signaling pathway in controlling intervertebral disk cell response to inflammatory and mechanical stressors. Phys Ther. 2016;96(5):704-711.
- Daniels J, Binch AA, Le Maitre CL. Inhibiting IL-1 signaling pathways to inhibit catabolic processes in disc degeneration. J Orthop Res. 2017;35(1):74-85.
- Kobori S, Miyagi M, Orita S, et al. Inhibiting IĸB kinase-β downregulates inflammatory cytokines in injured discs and neuropeptides in dorsal root ganglia innervating injured discs in rats. Spine (Phila Pa 1976). 2014;39(15):1171-1177.
- Nerlich AG, Weiler C, Zipperer J, Narozny M, Boos N. Immunolocalization of phagocytic cells in normal and degenerated intervertebral discs. Spine (Phila Pa 1976). 2002;27(22):2484-2490.
- Peng B, Hao J, Hou S, et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976). 2006;31(5):560-566.
- Chou PH, Shi P, Lin CL, et al. Small molecule antagonist of C-C chemokine receptor-1 can reduce disc inflammation in the rabbit model. 44th International Society for the Studies of the Lumbar Spine Annual Meeting; Athens, Greece. 2017.
- Hiyama A, Sakai D, Risbud MV, et al. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62(10):3036-3047.
- Kondo N, Yuasa T, Shimono K, et al. Intervertebral disc development is regulated by Wnt/β-catenin signaling. Spine (Phila Pa 1976). 2011;36(8):E513-518.
- Wang M, Tang D, Shu B, et al. Conditional activation of beta-catenin signaling in mice leads to severe defects in intervertebral disc tissue. Arthritis Rheum. 2012;64(8):2611-2623.
- Rannou F, Richette P, Benallaoua M, et al. Cyclic tensile stretch modulates proteoglycan production by intervertebral disc annulus fibrosus cells through production of nitrite oxide. J Cell Biochem. 2003;90(1):148-157.
- Yang X, Jin L, Yao L, Shen FH, Shimer AL, Li X. Antioxidative nanofullerol prevents intervertebral disk degeneration. Int J Nanomedicine. 2014;9:2419-2430.
- Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater. 2015;30:89-102; discussion 103.
- Suzuki S, Fujita N, Hosogane N, et al. Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Res Ther. 2015;17:316.
- Chen JW, Ni BB, Li B, Yang YH, Jiang SD, Jiang LS. The responses of autophagy and apoptosis to oxidative stress in nucleus pulposus cells: implications for disc degeneration. Cell Physiol Biochem. 2014;34(4):1175-1189.
- Kim KW, Ha KY, Lee JS, Rhyu KW, An HS, Woo YK. The apoptotic effects of oxidative stress and antiapoptotic effects of caspase inhibitors on rat notochordal cells. Spine (Phila Pa 1976). 2007;32(22):2443-2448.
- Li X, Phillips FM, An HS, et al. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine. 2008;33(24):2586-2595.
- Wuertz K, Quero L, Sekiguchi M, et al. The red wine polyphenol resveratrol shows promising potential for the treatment of nucleus pulposus-mediated pain in vitro and in vivo. Spine (Phila Pa 1976). 2011;36(21):E1373-1384.
- Kwon YJ. Resveratrol has anabolic effects on disc degeneration in a rabbit model. J Korean Med Sci. 2013;28(6):939-945.
- Gruber HE, Hoelscher G, Ingram JA, Chow Y, Loeffler B, Hanley EN Jr. 1,25(OH)2-vitamin D3 inhibits proliferation and decreases production of monocyte chemoattractant protein-1, thrombopoietin, VEGF, and angiogenin by human annulus cells in vitro. Spine (Phila Pa 1976). 2008;33(7):755-765.
- Kim JS, Ellman MB, Yan D, et al. Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. J Cell Physiol. 2013;228(9):1884-1896.
- Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204(1):47-54.
- Le Maitre CL, Freemont AJ, Hoyland JA. Human disc degeneration is associated with increased MMP 7 expression. Biotech Histochem. 2006;81(4-6):125-131.
- Seguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976). 2005;30(17):1940-1948.
- Seguin CA, Pilliar RM, Madri JA, Kandel RA. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine (Phila Pa 1976). 2008;33(4):356-365.
- Ellman MB, Kim JS, An HS, et al. Toll-like receptor adaptor signaling molecule MyD88 on intervertebral disk homeostasis: in vitro, ex vivo studies. Gene. 2012;505(2):283-290.
- Anderson DG, Izzo MW, Hall DJ, et al. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine. 2002;27(12):1291-1296.
- Sobajima S, Shimer AL, Chadderdon RC, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5(1):14-23.
- Mwale F, Masuda K, Pichika R, et al. The efficacy of link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res Ther. 2011;13(4):R120.
- Leckie SK, Bechara BP, Hartman RA, et al. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012;12(1):7-20.
- Brandt KD, Mazzuca SA, Katz BP, et al. Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2005;52(7):2015-2025.
- Krzeski P, Buckland-Wright C, Balint G, et al. Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res Ther. 2007;9(5):R109.