Zorica Buser and Jeffrey C. Wang
INTRODUCTION - SPINAL FUSION
Spine pathologies with a very complex interplay between neurological, bone and soft tissue components are some of the most common debilitating conditions worldwide. While the default approach for many conditions is conservative treatment, aggressive pathologies or trauma injuries require surgical attention. Spine arthrodesis is a very versatile approach that is used to treat a large array of spine pathologies, including degenerative conditions, deformity, trauma and tumors. Spine pathology and patient background will dictate the fusion approach, which can be anterior or posterior with certain modifications. In both approaches, bone decortication and bleeding are a must for successful graft incorporation and bone remodeling. In the posterior approach, transverse processes and facet joints are exposed and decorticated. While in the anterior approach, the cartilaginous endplates are scraped to expose the bony endplates. Another important difference between the two approaches is the type of force applied to the spine. In a posterior approach, the spine is exposed to tension forces requiring a graft with good osteobiologic properties to achieve a solid fusion. In an anterior procedure, the compression load requires a graft material with strong structural characteristics that can sustain the heavy load. Both approaches and their modifications are supported by a variety of spinal instrumentation products.
While there are constant improvements in the fusion techniques, non-union (pseudarthrosis) remains the main procedural complication. Studies have shown that depending on the type of grafting material, patient comorbidities, the approach and the number of levels, the pseudarthrosis rates in the lumbar and cervical spine can range from a few percent for a single level to >40% for multi-level procedures.1,2
FUSION BIOLOGY AND GRAFT OPTIONS
New bone formation is the key for successful spinal fusion and heavily depends on the local bone environment and the graft material. Although the initial stability is achieved by the spine instrumentation, the bone graft provides a foundation for bone remodeling that occurs over a longer period. Spinal fusion is a very complex process of molecular events that can be divided into three major phases as described by Boden et al.3 Decortication of transverse processes or endplates initiates the first stage, the inflammatory phase, which lasts for up to 3 weeks. During this phase, a wide array of inflammatory cells including macrophages, leukocytes and lymphocytes occupies the fusion site, and a large number of cytokines are secreted. Bone formation is initiated by membranous ossification. In the second stage, the reparative phase, initial steps of fusion mass formation and cancellous bone remodeling occur. This coincides with resorption of necrotic tissue, vascularization and progenitor cell differentiation. During this stage, bone formation migrates towards the central zone of the fusion site. Once the reparative phase is completed the remodeling phase begins and lasts up to 4 weeks. In this stage, the mature bone is formed, cartilaginous remnants dissipate and the bone marrow volume increases. The maturation of bone slows down for the outer zone and increases for the central zone; both reach a similar level at the end of 10 weeks.
These stages of spinal fusion shape expectations of a graft material and the definition of an ideal candidate. A grafting material can carry out the three stages of fusion only if it is: (1) osteogenic, providing mature osteoblasts and stem cells that will drive new bone formation; (2) osteoinductive, containing various growth factors that promote osteogenesis of the surrounding stem cells; and (3) osteoconductive, providing mechanical stability and scaffold pores large enough to assist neovascularization, cell migration and the ingrowth of bone. In addition to these properties, the ideal graft material should carry no risk of disease transmission
| Ideal Graft Characteristics | Donor Caracteristics | ||||
|---|---|---|---|---|---|
| Grafts | Osteoconductive | Osteoinductive | Osteogenic | Age/comorbidities | Disease transmission |
| Autologous bone | • | • | • | ♦ | |
| Allograft bone | • | • | ♦ | ♦ | |
| DMB | • | • | ♦ | ♦ | |
| Ceramics | • | ||||
| BMP | • | ||||
| BMA and cells | • | • | ♦ | ||
Of all available grafting materials, an autologous bone graft is the only option incorporating all three bone properties. It also provides immediate and long-term mechanical stability without risk of disease transmission (Table 7-1), but complications associated with harvesting and limited volumes have led to a reduction in the use of autograft material. To replace autografts, various other grafting options have been developed, including allografts (e.g., demineralized bone matrices (DBMs)), ceramics, growth factors, bone marrow aspirates (BMA), autogenous platelet concentrate, and mesenchymal stem cells (Table 7-1). Based on their capabilities, those graft options can be divided into three groups: graft extenders, substitutes or enhancers. These are discussed below in comparison to the ideal autograft.
Autologous Graft
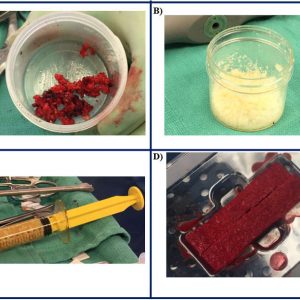
Autograft bone is the only grafting material containing osteoinductive factors (bone morphogenic proteins (BMPs), particularly BMP-2 and 7), an adequate matrix that enables osteoconduction and most importantly the stem cells and osteoblasts driving osteogenesis. The two primary types of autograft bone are cancellous and cortical (Fig. 7-1A). Depending on the harvest site, autografts can originate from local bone or extraspinal material such as an iliac crest bone graft (ICBG). Local bone is harvested during decompression from the lamina, facets, or processes, and its cortical nature provides immediate mechanical support. However, due to the small pore size of the cortical bone, the migration of cells and blood vessels is reduced, thus slowing down bone remodeling and diminishing the long-term stability of the fusion site. Its benefit is that no additional procedure or incision is needed for the harvest. In comparison, the iliac crest bone graft is cancellous bone with larger pore size and surface area which provides an ideal environment for vascularization and bone formation. The rapid bone remodeling occurring with ICBG grafts provides excellent mechanical stability, which compensates for the lack of compressive strength. Although in certain procedures ICBG is harvested in the same approach, most patients require an additional procedure for graft harvest.
The harvest of both local bone and ICBG can lead to major or minor complications, including infection, fracture, hernia, blood loss, vascular injury, the lengthening of stay and several others.4,5 While ICBG has always been seen as a graft with a higher incidence of complications, studies have shown conflicting data on postoperative pain at the donor site. Howard et al. compared the post-operative pain in patients undergoing posterior lumbar fusion with ICBG or BMP2.6 They did not find any significant differences between the two groups regarding post-operative pain. Furthermore, the number of levels and body mass index (BMI) were not associated with either the incidence or severity of post-operative pain. While this study has not found any differences when controlling for the number of levels fused and patient’s BMI, the patient’s demographics and surgery profile play an important role. Bone quality declines with age and patient comorbidities such as smoking, diabetes and several others that significantly affect fusion success. Another challenge with autografts is the amount of grafting material required for multi-level procedures. Currently, various graft extenders are used along with autograft material to carry out multi-level fusions. Comparing local bone to ICBG, Sengupta et al. reported similar fusion rates in single level posterior lumbar interbody fusion (PLIF).7 In the multi-level procedure, ICBG had three times higher fusion rates than local bone. However, there was no difference in the clinical outcomes with a local bone having less morbidity. Although the ICBG and local bone have been widely used in lumbar fusion, a recent systematic review found only three studies that met inclusion and exclusion criteria, all with a low level of evidence.8 Keeping that in mind, donor-site complications were higher in patients receiving ICBG. However, fusion rates, outcomes and pain were similar among two groups.
Allografts
Allografts are materials obtained from cadaveric tissue and are one of the most commonly used grafts in various procedures, including spinal fusion (Figure 7-1B). Allografts are prepared either through freezing or freeze-drying procedures, the latter having reduced biomechanical properties. Because they originate from cadaveric tissue, a critical step during preparation removes immune antigens and prevents any potential disease transmission. This process strips off cells and most of the bone growth factors leaving allografts with superior osteoconductive properties and lessor osteoinductive properties. The success of allografts are dependent on the type of bone and surgical procedure, which has led to their use as a graft extender or substitute. Similar to an autograft, allograft bone of cortical origin provides good mechanical stability, but, due to the trabecular density, bone remodeling is slow, and graft resorption is increased. On the other hand, corticocancellous allografts with a large surface area provide rapid bone remodeling and graft resorption, leading to long-term mechanical stability. Posterior fusion requires a grafting material that can sustain high tensile forces, and allografts have shown less favorable outcomes when used alone.9 As a graft extender, allografts have demonstrated high fusion rates in posterior spine surgeries for deformities. Patients with idiopathic scoliosis undergoing fusion with freeze-dried allograft extender had up to 97% fusion rates and minimal loss of correction, which is probably due in part to the young age of the patients.10 For the anterior interbody fusion, allografts are often used as substitutes to autograft due to their high resilience to a compressive load. A combination of femoral ring and cancellous allograft was used in patients undergoing anterior lumbar fusion, leading to 97% fusion rates at 6 to 12 months follow-up.11 The fusion rate of the structural allograft increases with the rigidity or stability of the construct, especially in the multi-level fusion cases. While allografts are available in large quantities and at low cost, the donor age and the presence of comorbidities remain challenging. Bone diseases such as osteoporosis or osteoarthritis can significantly reduce the mechanical properties of the allograft and lead to lower fusion rates. The most common forms of allografts include bone chips, strips or demineralized bone matrix (DBM).
Demineralized bone matrix (DBM)
Demineralized bone matrix is the most commonly used allograft in cervical and lumbar fusion. As with other allograft materials, it is obtained from cadaver tissue that is processed with several acidic washes and incubations removing cells, a majority of growth factors and the mineral components of the bone. The final product is osteoconductive and to a lesser extent osteoinductive. Its mechanical stability is due to collagen proteins (primarily Collagen I), some glycoproteins and calcium phosphate, while its osteoinductive property is driven by cytokines of the tumor growth factor beta (TGF-β) superfamily, especially BMP2, BMP7, and TGF-β. Donor age and comorbidities heavily influence the level of available growth factors. However, insulin growth factor 1 (IGF-1) and TGF-β are affected by aging to a lesser extent. Their presence plays an important role in osteoinduction by facilitating stem cell differentiation and recruitment. DBM powder is combined with various carriers (calcium sulfate, glycerol, alginate or hyaluronic acid) for easier delivery at the surgical site. The particle size and fiber orientation in DBM powders are determining factors in the graft’s osteoinductive capabilities. Particles that are smaller than 250µm have been correlated with a lower osteoinductive potential of the scaffold.12 The most common DBM forms include chips, putty paste, sheets and gel-filled syringes (Fig. 7-1C).
DBM has been extensively studied in animal models, including rodents, rabbits and canine. In a posterolateral fusion rabbit model, DBM gel was used in various ratios as an extender to ICBG.13 All groups achieved similar fusion rates at six weeks; a 3:1 ratio of DBM and ICBG has the most comparable rates to autograft alone. Furthermore, all groups with DBM had a greater level of trabecular bone formation compared to autograft alone. While animal models have demonstrated that DBM can be used successfully as a graft extender, several studies have pointed out the osteoinductive variation in DBM. Wang et al. compared three commercially available DBM products and found fusion rates varying from 0% for Dynograft DBM putty to 78% for Osteofill DBM paste.14 In a clinical setting, DBM has been extensively used as a graft extender with comparable fusion rates to autograft alone or other graft substitutes. In a PLIF prospective study, DBM Grafton gel had similar fusion rates and level of bone mineralization as ICBG alone at the 2-year follow-up (52% vs. 54%).15 Epstein et al. used DBM in combination with autograft in one and two-level PLF fusions.16 They saw an improvement in SF-36 scores in both groups and similar fusion rates in dynamic X-rays ranging from 96% to 98%. Furthermore, DBM has shown potential in combination with bone marrow aspirates (BMA), leading to overall fusion rates of 81.3% in a combined PLF and TLIF procedure.17 In addition to successful fusion, 72.5% of the patients had excellent or good functional outcomes, and the reported complications included only a few cases of pseudarthrosis, infection and hardware failure. However, it is important to emphasize that fusion outcomes are significantly impacted by the variability between commercial products and lot numbers caused by differences in DBM preparation and the donor’s age and comorbidities.
Ceramics
Ceramics are one of the few grafting materials with which the influence of patient age, comorbidities or disease transmission is not a risk factor. They are osteoconductive scaffolds lacking growth factors, osteoblast and stem cells. Since ceramics are not of cadaveric origin, they are easily obtainable in large amounts, and their costs are relatively low. From a structural perspective, they have a pore size that is similar to cancellous bone (up to 400 µm), which allows for cell and blood vessel migration. At the same time, their compressive strength (10 to 60 MP) is a fifth of the strength of cortical bone making them weak to a compression load. Another limitation of all ceramics is their brittleness. In spinal fusion, the drawbacks, similar to allografts, are a susceptibility to tensile forces in posterior fusion and a poor resistance to a compression load in the anterior approach leading to a need for instrumentation. With all their benefits and disadvantages, ceramics are good graft extenders. Furthermore, ceramics have been mixed with other graft extenders to complement each other and provide a substitute for autologous grafts. The most common ceramics are calcium phosphates, hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP). HA is an inert substance which remains present at the fusion site for up to a year, whereas TCP is degraded within the first two months. A large number of studies have shown their benefits as graft extenders with rigid fixation in both lumbar and cervical spine. Several studies have demonstrated solid rates of fusion when HA and β-TCP were used in posterolateral fusion. In a prospective single-center case matched study, patients underwent PLIF or PLF with pedicle screw with a mix of local bone and HA on one side and ICBG on the other side of the posterolateral gutter.18 At one year, the fusion rates were very similar between the HA and ICBG groups, 86.7% and 88.9% respectively. Furthermore, the fusion mass volume was greater in the hydroxyapatite/local graft group with the rates being 2.35ml for HA and 1.31ml for the ICBG group. The fusion success of HA and β-TCP extends to deformity cases, which require extensive volumes of the bone graft. Lerner and co-workers reported good results with the use of β-TCP as a bone extender in posterior fusion for adult idiopathic scoliosis.19 They reported no significant differences between the two groups and the overall loss of correction was 2.6 degrees for the β-TCP group and 4.2 degrees for the ICBG group. One of the patients who received β-TCP underwent revision surgery (1/20), and four patients in the ICBG group reported donor site pain (4/20).
Other graft substitutes include Silicate-substituted calcium phosphate (SiCaP), calcium sulfate and mixtures of collagen and HA. Calcium sulfate and SiCaP have been used in lumbar fusion as graft extenders showing variable fusion rates, graft subsidence and non-union. Also, calcium phosphate has been linked to post-operative complications, including infection and wound complications. Although synthetic grafting materials have been used widely in spinal fusion procedures, in a recent systematic review, only 19 studies qualified after inclusion and exclusion criteria.20 The analysis demonstrated that although there was no difference between the groups in fusion rates, functional outcomes or complications, the quality of evidence was low or insufficient due mainly to the high risk of bias and small sample size. This included HA, β-TCP, calcium sulfate and HA, and collagen.
GROWTH FACTORS
Autograft and a few allograft options possess osteoinductive capabilities that arise from growth factors. However, due to the patient/donor age and comorbidities, the levels of the osteogenic growth factors vary as well as their efficacy. On the other hand, recombinant growth factors are available in large quantities, and their efficiency is not compromised. Furthermore, they lack immunogenic properties and are free of impurities. The transforming growth factor β (TGF-β) superfamily harbors several osteogenic growth factors, BMPs in particular. Bone morphogenetic proteins play an important role in development, cell differentiation and tissue morphogenesis. They are one of the key elements in regulating both normal skeletogenesis as well as several skeletal pathologies, including fibrodysplasia ossificans progressiva (FOP), Marfan syndrome, Loeys-Dietz syndrome and osteoarthritis. As for osteogenesis, BMPs (in particular BMP2 and BMP7) induce bone formation by stimulating stem cell differentiation, angiogenesis and alkaline phosphatase. It is important to note that not all BMPs are involved in all steps of osteogenesis and that there is an osteogenic hierarchy within BMPs with BMP2, 7 and 9 having the leading roles. BMP-induced osteogenesis is carried out through a complex interplay of several signaling pathways, including Smad (sma and mothers against decapentaplegic), Shh, Wnt/β-catenin, MAPKs and PI3K/AKT. The osteogenic potential of BMP2 and BMP7 was evaluated in a large number of in vitro and in vivo studies with a wide range of working concentrations (in vitro BMP2: 100-400ng/ml; in vivo: depending on animal model 0.2-1.5mg/ml). Studies have shown strong osteogenesis resulting in stimulating alkaline phosphatase, gene expression, cell differentiation and high fusion rates.21 However, BMPs are soluble factors that tend to diffuse away from the fusion site leading to a reduction in their osteoinductive potential. For this reason, they are always combined with a carrier that confines them to the location where they are needed and controls their release over time. Depending on the location and surgical approach, BMP2 is currently combined with autograft, DBMs, collagen, ceramics and polylactic acid (PLA) (Figs. 7-2 and 7-3).
In a clinical setting, only the recombinant human rhBMP2 and rhBMP7 have been approved for patient use. A multi-center study done by Vaccaro et al. led to Food and Drug Administration (FDA) approval of rhBMP7 (OP-1) for revision posterolateral lumbar surgeries.22 At the two-year follow-up, they found that patients treated with OP-1 had better post-operative outcomes and higher fusion rates compared to patients who received autograft (55% vs. 40% respectively). Burkus et al. performed one of the first studies on rhBMP2 that generated data for FDA approval of rhBMP2 in ALIF procedures. Patients with degenerative lumbar disc disease underwent ALIF procedure with tapered threaded fusion cages.23 Patients were randomly assigned into two groups, BMP2 with collagen sponge or ICBG. At 24 months, the clinical outcomes were similar between the two groups. However, fusion rates were higher in the BMP2 group compared to ICBG (94.5% vs. 88.7%). FDA approval and the very high fusion rates have led to off-label use of BMP2. Widespread use of rhBMP2 has introduced drawbacks, including complications, high costs and large variability in the dosing. Some of the reported complications include inflammation, prevertebral swelling and airway obstruction, osteolysis, ectopic bone formation, retrograde ejaculation in male, increased risk of malignancy, and others. A recent meta-analysis found that for an identical procedure the rhBMP2 concentration varied in a range of up to 10-fold between different studies with no real benefits associated with a higher dose and, thus, stressed the need for better BMP dosing recommendations.24
Although BMP2 has shown great potential, several other growth factors have been identified, and their role in osteogenesis is actively being investigated. These include BMP9, GDF-5, LIM-1 and several others.
BONE MARROW ASPIRATES (BMA) AND STEM CELL-BASED OPTIONS
Bone Marrow Aspirates represent a great source of progenitor cells and growth factors. Although they fully lack osteoconductivity and mechanical support, they can provide a good graft substitute in conjunction with an appropriate carrier. In addition, the BMA harvest leads to fewer complications associated with the donor site morbidity compared to ICBG. Due to the low number of viable progenitor cells in an unfractionated BMA (1 in 50,000), various techniques and instrumentation have been developed. Once concentrated, BMA is combined with a carrier (DBM or collagen I sponge) that provides mechanical stability (Fig. 7-1D). Various clinical studies have demonstrated similar fusion rates between BMA+carrier and ICBG in single-level procedures. In a systematic review, Khashan et al. compared fusion rates and clinical outcomes in studies using BMA or stem cells in conjunction with a carrier (ceramics, DBM or collagen) vs. ICBG or local autograft. In general, the overall body of evidence was weak for all identified studies.25 When compared to local bone, BMA in combination with a collagen sponge or ceramic produced similar fusion rates. On the other hand, when BMA was compared to ICBG in all four identified studies, fusion rates were lower in the BMA group compared to ICBG.25
Another strategy to improve the potential of BMA is the isolation and expansion of progenitor stem cells and osteoblasts. Mesenchymal stem cells (MSCs) are a self-renewing and pluripotent cell line with anti-inflammatory properties. Bone marrow stem cells have been used in a large number of cell culture and animal models showing a great differentiation potential towards osteoblasts when stimulated with growth factors. In a clinical setting, Gan et al. used concentrated MSCs from BMA in combination with β-TCP for posterior spinal fusion and reported 95.1% fusion rates at 34.5 months.26 Although isolated stem cell populations provide a great concentrated pool of osteogenic potential, the challenges with this approach are technical and logistic, including in vitro expansion, potential contamination, shelf life, cell viability after defrosting and the need for another procedure for harvest.
PEARLS AND PITFALLS
- Each bone graft carries risks and benefits, and the right choice will strongly depend on the patient’s background and the type of surgery.
- It is important to remove the soft tissue from a local bone to facilitate osteogenesis.
- Minimally modified stem-cell therapies do not require FDA approval and therefore lack pre-clinical studies.
- Platelet gels contain platelets and growth factors. However, the current literature demonstrated lower fusion and higher non-union rates, deeming them not suitable for spinal fusion.
- BMPs play an important role in osteogenesis and possibly tumorogenesis.
SUGGESTED READING
- Hsu WK, Goldstein CL, Shamji MF, Cho SK, Arnold PM, Fehlings MG, Mroz TE. Novel osteobiologics and biomaterials in the treatment of spinal disorders. Neurosurgery. 2017;80(3S):S100-S107.
- Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine (Phila Pa 1976). 2010;35(9 Suppl):S86-104.
- Rao PJ, Pelletier MH, Walsh WR, Mobbs RJ. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg. 2014;6(2):81-89.
REFERENCES
- Chun DS, Baker KC, Hsu WK. Lumbar pseudarthrosis: a review of current diagnosis and treatment. Neurosurg Focus. 2015;39(4):E10.
- McAnany SJ, Baird EO, Overley SC, Kim JS, Qureshi SA, Anderson PA. A meta-analysis of the clinical and fusion results following treatment of symptomatic cervical pseudarthrosis. Global Spine J. 2015;5(2):148-155.
- Boden SD, Schimandle JH, Hutton WC, Chen MI. 1995 Volvo Award in basic sciences. The use of an osteoinductive growth factor for lumbar spinal fusion. Part I: Biology of spinal fusion. Spine. 1995;20(24):2626-2632.
- Gruskay JA, Basques BA, Bohl DD, Webb ML, Grauer JN. Short-term adverse events, length of stay, and readmission after iliac crest bone graft for spinal fusion. Spine. 2014;39(20):1718-1724.
- Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3(3):192-195
- Howard JM, Glassman SD, Carreon LY. Posterior iliac crest pain after posterolateral fusion with or without iliac crest graft harvest. Spine J. 2011;11(6):534-537.
- Sengupta DK, Truumees E, Patel CK, et al. Outcome of local bone versus autogenous iliac crest bone graft in the instrumented posterolateral fusion of the lumbar spine. Spine (Phila Pa 1976). 2006;31(9):985-991.
- Tuchman A, Brodke DS, Youssef JA, et al. Iliac crest bone graft versus local autograft or allograft for lumbar spinal fusion: a systematic review. Global Spine J. 2016;6(6):592-606.
- Jorgenson SS, Lowe TG, France J, et al. A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patient. A minimum of 1-year follow-up in 144 patients. Spine (Phila Pa 1976). 1994;19:2048-2053.
- Knapp DR Jr, Jones ET, Blanco JS, Flynn JC, Price CT. Allograft bone in spinal fusion for adolescent idiopathic scoliosis. J Spinal Disord Tech. 2005;18 Suppl:S73-76.
- Kozak JA, Heilman AE, O'Brien JP. Anterior lumbar fusion options. Technique and graft materials. Clin Orthop Relat Res. 1994;(300):45-51.
- Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. 2012 Demineralized bone matrix in bone repair: history and use. Adva Drug Deliv Rev. 2012;64(12):1063-1077.
- Morone MA, Boden SD. Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel. Spine (Phila Pa 1976). 1998;23:159-167.
- Wang JC, Alanay A, Mark D, et al. A comparison of commercially available demineralized bone matrix for spinal fusion. Eur Spine J. 2007;16(8):1233-1240.
- Cammisa FP Jr, Lowery G, Garfin SR, et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine (Phila Pa 1976). 2004;29(6):660-666.
- Epstein NE1, Epstein JA. SF-36 outcomes and fusion rates after multilevel laminectomies and 1 and 2-level instrumented posterolateral fusions using lamina autograft and demineralized bone matrix. J Spinal Disord Tech. 2007;20(2):139-145.
- Ajiboye RM, Eckardt MA, Hamamoto JT, Plotkin B, Daubs MD, Wang JC. Outcomes of demineralized bone matrix enriched with concentrated bone marrow aspirate in lumbar fusion. Int J Spine Surg. 2016;10:35.
- Lee JH, Hwang CJ, Song BW, et al. A prospective consecutive study of instrumented posterolateral lumbar fusion using synthetic hydroxyapatite (Bongros-HA) as a bone graft extender. J Biomed Mater Res A. 2009;90(3):804-810.
- Lerner T, Bullmann V, Schulte TL, Schneider M, Liljenqvist U. A level-1 pilot study to evaluate of ultraporous beta-tricalcium phosphate as a graft extender in the posterior correction of adolescent idiopathic scoliosis. Eur Spine J. 2009;18(2):170-179.
- Buser Z, Brodke DS, Youssef JA, et al. Synthetic bone graft versus autograft or allograft for spinal fusion: a systematic review. J Neurosurg Spine. 2016;25(4):509-516.
- Campana V, Milano G, Pagano E, et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 2014;25(10):2445-2461.
- Vaccaro AR, Anderson DG, Patel T, et al. Comparison of OP-1 Putty (rhBMP-7) to iliac crest autograft for posterolateral lumbar arthrodesis: a minimum 2-year follow up pilot study. Spine (Phila Pa 1976). 2005;30:2709-2716.
- Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech 2002;15(5):337-349.
- Hofstetter CP, Hofer AS, Levi AD. Exploratory meta-analysis on dose-related efficacy and morbidity of bone morphogenetic protein in spinal arthrodesis surgery. J Neurosurg Spine. 2016;24(3):457-475.
- Khashan M, Inoue S, Berven SH. Cell based therapies as compared to autologous bone grafts for spinal arthrodesis. Spine (Phila Pa 1976). 2013;38(21):1885-1891.
- Gan Y, Dai K, Zhang P, et al. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29(29):3973-3982.