Daniel Jonsson and Kjell Olmarker
INTRODUCTION
The acute onset of radicular pain in the lower extremity, commonly referred to as sciatica, is a phenomenon that has had documented impact on human lives for as far back in time as history allows us to review.1 Ancient Egyptians and Greeks, Hippocrates included, introduced an organic rather than spiritual hypothesis to the genesis of symptoms and even suggested a relationship between spinal disorders and sciatic pain. Pioneering work in the late 19th/early 20th century by Laségue, Schmorl, Mixter, Barr and others gave rise to the theory of a “ruptured intervertebral disc” putting pressure on nerves, which in turn causes pain. The pressure was long believed to be the sole cause of lumbar radiculopathy, but extensive research during the last decades has provided a more complex view of its pathophysiology. It is now evident that a chemical factor, acting to sensitize the nerve root and thus making it susceptible to pressure, is required for the onset of pain.
This chapter aims to review current knowledge of the mechanisms behind lumbar radiculopathy with emphasis on disc herniation, as well as give an insight into what implications ongoing translational research could have in the not so distant future.
Definitions and Symptomatology
Lumbar radiculopathy, as defined herein, is a condition affecting one or more nerve roots of the lumbar spine typically caused by a lumbar disc herniation. The symptoms of lumbar radiculopathy can be divided into two categories: pain and nerve dysfunction. These categories commonly coincide, but their relative contribution to discomfort varies between patients. The pain is typically of a sharp and/or burning quality and follows the nerve root(s) area of sensory innervation in the lower extremity. Nerve dysfunction can be further divided into the loss of motor and/or sensory modalities, causing muscle weakness and/or atrophy and sensory disturbances.
The pathophysiology of lumbar radiculopathy involves a combination of pressure and chemical factors. The following sections will review the evidence of the pathophysiology, with the aim to elucidate the respective contribution of pressure and chemical factors to the different symptoms in lumbar radiculopathy.
PRESSURE
Even though pressure was considered the sole cause of symptoms in lumbar radiculopathy during most of the 20th century, only limited experimental research was performed to elucidate the mechanisms behind this hypothesis. However, with the introduction of new experimental models in the late 80s to mid-90s, this changed. Multiple studies have identified circulatory compromise, decreased transport of nutrients resulting in neuro-ischemia, as an important mechanism through which pressure contributes to lumbar radiculopathy.
Nerve Conduction
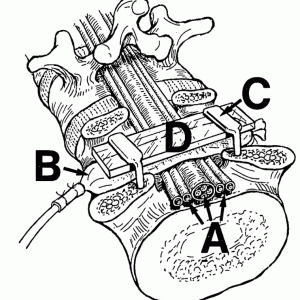
Early experimental studies by Gelfan and Tarlov in 1956 and Sharpless in 1975 indicated that pressure on nerves induced a decrease in nerve impulse conduction, and this was more pronounced in nerve roots than in peripheral nerves.2,3 In the late 80s, a new experimental pig model was introduced that allowed for gradual compression of cauda equina nerve roots on one or more spinal segments with the simultaneous visual observation of the pressurized and adjacent segments (Fig. 8-1).4 This model allows for evaluation of nerve conduction velocity, blood flow, edema formation and subsequently histological changes of the nerve roots of the cauda equina at given pressure levels. Nerve conduction velocity in this model of acute compression was found to be decreased at pressure levels of 100 and 200 mmHg, but not at 50 mmHg, and the observed decreases were more prominent with immediate (<0.1 seconds) onset of pressure as compared to a gradual increase over 20 seconds.5 To study the effects of chronic compression, Konno et al. created a new dog model where an inflatable balloon was introduced under one or more laminae.6 This model allows for both acute-, chronic- and acute-on-chronic in vivo compression studies. Also, physiological differences in pressure caused by the animal’s movements also contribute to making this model more relevant to the clinical setting. Studies of acute compression in this model produced similar results to those in the pig model by Olmarker et al, i.e. pressure levels of about 100 mmHg are required to induce an acute decrease in nerve conduction velocity.7 Although, one week after application of an initial pressure of just 10 mmHg, a decrease in nerve conduction velocity was observed.8 Interestingly, in the same study, it was found that acute compression of an additional 100 mmHg after one week of chronic compression caused a lesser decrease in nerve conduction velocity compared to animals who did not receive the initial compression. This suggests that chronically compressed nerve roots can adapt and develop a resistance to additional acute compression.
FIGURE 8-1. Schematic drawing of an experimental nerve root compression model. The cauda equina (A) is compressed by an inflatable balloon (B) that is fixed to the spine by two L-shaped pins (C) and a plexiglas plate (D).
The effects of two-level compression have also been evaluated in these experimental models. In the pig model of acute compression, it was shown that pressure applied to two adjacent segments induced a more pronounced reduction in nerve conduction velocity as compared to single-level compression.9 Interestingly, the decrease was even more pronounced when the distance between the compressed segments was increased. This indicates that the effects on nerve conduction velocity are caused by circulatory compromise (see below). However, in the dog model of chronic compression, no difference in nerve conduction velocity was observed between one- and two-level compression after one week, but the recovery over time that was observed in single-level compression was less pronounced in the two-level compression group.10
It is thus evident that pressure applied to nerve roots can cause a decrease in impulse conduction velocity, and that pressure levels required to induce these effects are high in the acute setting and low in the chronic setting. The mechanisms of the later are likely the most relevant for degenerative disorders of the spine, including both disc herniation and spinal stenosis, and thus for lumbar radiculopathy.
Circulation and Nutrition Transport Failure
Reduced blood flow, subsequent ischemia and/or nutrition transport failure to the nerve tissue are the most likely mechanisms by which pressure can impair nerve functioning. In addition to the blood supply, usually from radicular arteries, nerve roots also have a significant supply of nutrients from cerebrospinal fluid (CSF).11 This, along with the lack of supportive tissue such as epi- and perineurium, is believed to contribute to the high susceptibility to nerve dysfunction caused by both pressure and chemical factors as compared to that of peripheral nerves.
Using the above-described pig model, Olmarker et al. found that the mean pressure required to stop the capillary blood flow in the nerve roots of the cauda equina was 40 mmHg.4 However, even at low-pressure levels (5-10 mmHg), a venular occlusion was observed. This was in turn believed to cause reduced capillary blood flow in the nerve tissue due to retrograde stasis. The hypothesis that even low-pressure levels can induce a reduction in blood flow is strengthened by studies in the above-mentioned dog model of chronic compression, where it has been found that blood flow in the nerve tissue decreases one week after application of an initial pressure of just 10 mmHg.12 The observation that low-pressure levels cause reduced blood flow in both acute and chronic settings, and that low pressure over time has been shown to induce nerve dysfunction as measured by nerve conduction velocity, are in line with the hypothesis that a pressure-induced chronic compromise to the circulation of the nerve root causes neuro-ischemia and/or reduced nutrient transport to the nerve root, which over time leads to nerve dysfunction. This hypothesis is further strengthened by two-level compression models. In a previously mentioned study by Olmarker et al., the impairment in nerve conduction velocity was greater with increased length between the compressed segments. Since the greater distance between compression sites means that a longer segment of the cauda equina is exposed to circulatory compromise, while the segment of the cauda equina that is pressurized stays the same, these results strengthen the hypothesis that the pressure-induced effect on nerve root conduction velocity is the result of circulatory compromise.9,13 A decrease in nutrient supply to the nerve root has also been demonstrated even at low-pressure levels (10 mmHg) with insufficient or no compensatory mechanisms, e.g. diffusion from CSF.14 Pressure has also been shown to induce edema formation in nerve roots, especially in the segments tightly adjacent to the compressed site,15 which may contribute to circulatory compromise.
Pressure and Pain
For obvious reasons, pain is more difficult to assess in experimental settings as compared to nerve conduction velocity and other objective measurements of nerve function, although some experimental studies have been performed with the aim to elucidate if pressure alone can cause pain. Kawakami et al. found that ligation of nerve roots per se did not induce changes in behavior associated with pain in the rat, but ligation using a ligature that also induced chemical irritation (chromic gut suture) did.16 Numerous experimental studies in different animal models have produced similar results.17,18 It thus seems evident that pressure alone is not the sole cause of pain in lumbar radiculopathy.
Implications and Clinical Correlations
Although they have some weaknesses, the above mentioned experimental studies have greatly contributed to our understanding of the pressure-induced effects of conditions such as disc herniation and spinal stenosis. For example, the blood flow of the nerve roots may vary due to the upright posture of humans as compared to the experimental models with animals typically in the prone position. The onset time of pressure in degenerative conditions such as spinal stenosis where a progressive narrowing of the spinal canal usually begins many years before the onset of symptoms is also difficult to reproduce.
It seems evident that pressure alone, when applied to nerve roots of the lumbar spine, causes mainly nerve dysfunction, not pain. This correlates with clinical observations. In a pioneering study, Kuslich et al. described patients’ responses to stimuli of various structures, including both healthy nerve roots and nerve roots in proximity/contact with a herniated disc, during open spine surgery under local anesthesia.19 They concluded that pressure on healthy nerve roots typically caused numbness and/or weakness, not pain, whereas pressure on nerve roots with contact to a herniated disc usually caused radiating pain. This strongly supports that pressure alone is not the sole cause of radiating pain in lumbar disc herniation with radiculopathy.
It is also recognized that reduced blood flow, with subsequent neuro-ischemia and lack of nutrients, is the most important pathophysiological mechanism through which pressure induces nerve dysfunction. This knowledge has had limited clinical implications, primarily in lumbar spinal stenosis. Medical treatment with prostaglandin E1, a vasodilator and inhibitor of platelet activation, has been shown to increase blood flow in lumbar nerve roots and improve symptoms in lumbar spinal canal stenosis.20 Although, due to lack of larger, high-quality, long-term efficacy and safety studies, this treatment has market approval only in a few Asian countries and is thus not widely available. No such medical treatment exists for lumbar radiculopathy in disc herniation. However, there are some interesting clinical observations regarding the relationship between pressure and symptoms in disc herniation. For example, nerve root pressure measured during surgery for disc herniation has been found to correlate with symptoms of nerve dysfunction, but not with the degree of straight leg raising.21 This is in line with several subsequently published papers, where the size of disc herniation and/or degree of nerve root compression on MRI correlates poorly with the severity of symptoms.
To conclude, pressure on nerve roots seems to induce nerve dysfunction due to reduced blood flow, subsequent relative ischemia and lack of nutrients in the nerve tissue. Pressure alone does not seem to be a significant cause of pain but contributes to pain in the presence of one or more chemical factor, acting to sensitize the nerve root. This is discussed below.
CHEMICAL FACTORS
The hypothesized importance of one or more biologically active substances, or chemical factors, independent of pressure in the pathophysiology of lumbar radiculopathy has been suggested in several articles from early on but was not investigated further until the early 90s. Due to the typical proximity of disc tissue to the symptomatic nerve root in lumbar radiculopathy caused by disc herniation, the disc hernia tissue is the likely source of such a chemical factor. The following will review the biological effects of disc tissue, mainly nucleus pulposus (NP), on nerve tissue from a macroscopic level down to subcellular mechanisms, through which mechanisms these effects are mediated and how these are believed to contribute to symptoms in lumbar radiculopathy.
Physiological Effects of Disc Tissue on Nerve Roots
The first proper experimental studies of the effects of disc tissue on nerve roots were performed by Olmarker et al. in the early 90s.22 Autologous NP placed epidurally over the cauda equina in pigs was found to reduce nerve conduction velocity as well as structural axonal changes, both consistent during the one-week study period. The axonal changes were focal in their distribution and were thus not likely the sole cause of the functional changes observed. This hypothesized lack of correlation between the structural and the functional changes observed was confirmed in subsequent studies, where for example freezing of the nucleus pulposus before application as well as treatment with methylprednisolone reduced the functional changes induced by NP but still showed similar structural changes.23,24 Application of NP to a nerve root has also been shown to increase endoneurial fluid pressure and reduce blood flow,25 and vital microscopy of blood flow of the hamster cheek-pouch following injection of a suspension of NP revealed both an increased vascular permeability and thrombosis formation.26
Experiments aiming to elucidate if NP applied on nerve tissue alone can induce pain have shown inconclusive results. Olmarker et al. found that NP applied to a nerve root and its dorsal root ganglion (DRG) did not induce changes in thresholds for mechanical or thermal stimuli.27 However, it was found that the combination of mechanical deformation and NP exposure of the nerve root-induced pain, but mechanical deformation alone did not. This was subsequently reproduced in similar experimental designs in the rat, but in a more recent study by Kawakami et al., it was shown that NP alone, when applied on the DRG, was enough to induce increased allodynia as well as gait abnormalities.28 It has also been shown that application of NP causes increased excitability and mechanical hypersensitivity in the DRG,29 as well as increased nociceptive transmission to the dorsal horn suggestive of hyperalgesia.30
It thus seems evident that NP acts to sensitize the nerve root to produce pain upon mechanical deformation and/or pressure, but also that NP likely has an inherent ability to induce pain without mechanical deformation. These effects, however, are likely related to subcellular mechanisms rather than structural changes in the nerve, and although not yet well understood, several such possible mechanisms have been investigated during the last decade. For example, studies have shown that application of NP to a DRG induces changes in expression of different ion channels in the DRG, such as acid-sensing ion channel 3 (ASIC3)31 and other voltage-gated sodium channels,32 which are likely to contribute to hyperexcitability and thus sensitization of the nerve roots. Another possible subcellular mechanism involves serotonergic transmission. Serotonin (5-HT) is a monoamine known to be involved in nociception, both by directly excite sensory nerve fibers and possibly also as a modulator of signal transmission at the spinal level through gate control mechanisms. Application of NP to the DRG in the rat has been shown to affect the expression of different 5-HT receptor subtypes in the DRG, and direct application of 5-HT to the DRG induces pain-related behavior.33 Treatment with a 5-HT2A receptor subtype antagonist was shown to reduce NP-induced hyperalgesia in the rat, but a clinical study using the same antagonist showed limited efficacy in treating symptoms of disc herniation, similar to that of treatment with NSAIDs.34 Also, the selective serotonin reuptake inhibitor paroxetine has also demonstrated efficacy in treating NP-induced hyperalgesia in the rat,35 which is contradictory. 5-HT thus seems to be involved in lumbar radiculopathy, but its role is complex and not well understood.
Mediators of NP-induced Physiological Effects
Inflammatory mechanisms in the pathophysiology of lumbar radiculopathy were frequently suggested long before the intrinsic effects of disc tissue on nerve roots were known. When experimental data demonstrating these effects first emerged, studies to assess if these effects could be altered by immunomodulatory drugs were therefore promptly designed. It was shown that the NP-induced reduction in nerve conduction velocity was substantially reduced by high-dose intravenous administration of methylprednisolone.24 In another study using the same model, it was shown that prednisolone also reduced the increase in vascular permeability induced by NP.36 Both studies suggest that the effects may be induced by an inflammatory reaction. The structural changes of the nerve roots were however not prevented by methylprednisolone, further emphasizing the lack of correlation between structural and functional changes in experimental models of lumbar radiculopathy.
To further assess which component of the inflammatory reaction was responsible for the observed effects, tumor necrosis factor-α (TNF) was chosen as an investigatory target since its known effects on nerve tissue and blood vessels closely resembled that of NP. TNF was identified in the cells of NP in pigs using immunohistochemistry, and inhibition of TNF partially blocked the NP-induced reduction in nerve conduction velocity described above.37 This was the first time a specific substance was suggested to be the mediator of the NP-induced physiological effects. TNF is also involved in other NP-induced effects, such as increased nociceptive transmission in the dorsal horn38 and the histologically observed damage and apoptosis in the dorsal root ganglion following the application of NP.39,40 TNF is also involved in the NP-induced changes in serotonergic transmission discussed above.41,42 It has further been shown to cause a decrease in nerve conduction velocity.43 TNF inhibition has been found to reduce NP-induced vascular compromise in the pig,44 prevent NP-induced increased nociceptive transmission in the dorsal horn,45 and reduce NP-induced histological damage to the dorsal root ganglion.39 TNF inhibition also prevented changes in spontaneous pain behavior in a rat model of disc herniation.46 Due to this and other compelling experimental data on the involvement of TNF in lumbar radiculopathy, clinical trials to evaluate TNF inhibitors in the treatment of sciatica have been performed. These are discussed later in this chapter.
Numerous experimental and clinical studies have subsequently suggested the involvement of pro-inflammatory cytokines other than TNF in the pathophysiology of lumbar radiculopathy as well. For example, IL-1β can also be identified in rat NP which may have importance for the reduction of nerve conduction velocity and induction of hyperalgesia,43,47 possibly augmenting the effects of TNF.48 NP has also been found to increase concentrations of IL-6 and INF-γ when applied to the DRG,49 and application of recombinant IL-6 to the DRG induced production of TNF at the site and caused apoptosis in the cells of the DRG.50
It thus seems evident that not only TNF but also other pro-inflammatory cytokines may exert pathophysiologically important effects in lumbar radiculopathy. The inert content of pro-inflammatory cytokines in the NP alone is unlikely to contribute to long-lasting symptoms in patients since TNF has a short half-life. It is thus reasonable to assume that an ongoing inflammatory process occurs which maintains the production of cytokines contributing to symptoms. An important question then remains to be answered, namely what drives the inflammation? This is not yet clearly defined, but several mechanisms have been considered. One of particular interest is the possibility of an autoimmune reaction to NP. For almost as long as inflammation has been suggested in the pathophysiology of lumbar radiculopathy, the possibility of an autoimmune reaction against the NP has been suggested as well. This hypothesis is commonly backed in the literature by arguments regarding the avascular and contained anatomy of the NP of the disc, which thus is not exposed to the immune system. When a disc herniation occurs, the herniated disc tissue is exposed to the vascularized structures in the spinal canal which allow the immune system to reach the NP, which in turn contains antigenic components triggering an autoimmune response. There is some evidence supporting this hypothesis. Immunoglobulins were identified in healthy canine NP as early as the mid-80s.51 The presence of autoantibodies in herniated NP, mainly in the pericellular capsule, in surgical samples from patients undergoing disc hernia surgery, has later been confirmed.52 Autoantibodies against extracellular matrix proteins of the NP have also been observed in degenerated intervertebral discs.53 Experimental data suggests that NP attracts and activates both T and B cells.54,55 It thus seems evident that the NP may trigger an autoimmune response, but the importance of such a reaction is not well understood.
However, there are some limitations to the hypothesis of autoimmunity. For example, the development of immunologic tolerance to self is not primarily dependent on circulating antigens. Central tolerance is also achieved by promiscuous gene expression by epithelial cells in the thymus, thus exposing the immature T cells to self-antigen and preventing the escape of autoreactive T cells to the periphery through negative selection. This system also needs to fail for NP-reactive T cells to enter the circulation, and the avascular and contained nature of the healthy NP can thus not in itself be considered the cause of autoimmunity. Differences in genetic predisposition to fail to develop central tolerance to the NP can be hypothesized to contribute to the hereditary component of the condition. Another limitation is that, upon first exposure to a new antigen, the immunologic response takes some time to develop. It is, therefore, unlikely that the early NP-induced physiologic effects on nerves observed in experimental studies are caused by an immunologic response. However, it can be hypothesized that the cytokine content of the NP gives it an inert ability to induce these early effects and that the immunologic response may be important for chronic symptoms, making it relevant to the clinical scenario.
Mechanisms other than inflammation through which NP may induce the physiological changes observed include the direct neurotoxic effect of components of the NP and vascular impairment with subsequent neuro-ischemia. The latter is supported by the observed changes in blood flow induced by NP discussed above. Also, the focal structural changes observed in the nerve roots following application of NP are mainly found in the center for the nerve, resembling the typical damage distribution caused by arterial infarction. These mechanisms likely overlap with and/or are in part the result of an inflammatory reaction.
To conclude, pro-inflammatory cytokines such as TNF act to sensitize the nerve root and contribute to symptoms of both nerve dysfunction and radicular pain in lumbar radiculopathy. Components of the NP may induce an inflammatory reaction that is likely responsible for the production of these cytokines, and an autoimmune reaction to components of the NP may contribute to this reaction. The importance of different drivers of the inflammatory reaction, mainly an autoimmune response, needs to be further elucidated.
Implications and Clinical Correlations
Different TNF inhibitors have been evaluated in clinical trials for the treatment of sciatic pain in disc herniation. In spite of compelling experimental data suggesting both a key pathophysiologic role for TNF and the therapeutic potential of its inhibition of NP-induced physiological effects, these studies have not been conclusive.56 Initial, smaller studies showed promising efficacy, but subsequent larger randomized studies have often failed to show efficacy. Therefore, TNF inhibition has not become an established treatment for lumbar radiculopathy in disc herniation. In a wider perspective, this incongruity between experimental and clinical data highlights the heterogeneity and complexity of lumbar radiculopathy and the difficulty in reproducing the condition in experimental models. New experimental approaches will likely be needed to successfully identify, evaluate and develop novel drug candidates for the treatment of lumbar radiculopathy. Given the growing evidence that pro-inflammatory cytokines other than TNF are involved in lumbar radiculopathy, it is probable that mechanisms upstream of these cytokines will be targets for treatment. Defining the drivers of inflammation in disc herniation and lumbar radiculopathy could, therefore, be an important next step towards developing new, more efficient medical treatment modalities.
MORPHOLOGY OF DISC HERNIAS
Since NP seems to be the driver of inflammation in lumbar radiculopathy caused by disc herniation, it is also of interest to discuss the morphology of disc hernias. The histological composition of the herniated tissue in disc herniation has been assessed in several studies. Kokubo et al. performed both histological and immunohistochemical analyses of cervical herniated discs resected en bloc from close to 200 patients.57 The en bloc resection during anterior cervical surgery is expected to be a substantially better pre-condition to assess the morphology of a hernia as a whole compared to the fragmented tissue typically obtained during conventional posterior lumbar discectomy. Kokubo et al. found that the majority of the hernias consisted of not only herniated disc material but also of macrophage-infiltrated granulation tissue commonly surrounding the herniated NP. Other common findings were neovascularization and the expression of TNF, matrix metalloproteinase 3, basic fibroblast growth factor and vascular endothelial growth factor. Most findings were particularly abundant in extruded and sequestrated hernias compared to protrusions. Since these findings were from cervical hernias, some caution needs to be taken when extrapolating the findings to lumbar hernias. However, several smaller and/or more limited assessments of extirpated disc hernia tissue taken during lumbar discectomy have reported similar findings.58-60
Several longitudinal studies using medical imaging modalities, primarily MRI, to investigate the progression of the size of lumbar disc hernias have been published. They indicate a more dynamic progress than what was previously considered, with spontaneous regression being a common observation. For example, Jensen et al observed spontaneous regression in the vast majority (75-100%) of broad-based protrusions, extrusions and sequestrations, but less so in focal protrusions (35%) and bulges (3%) in a prospective 14-month follow-up study of a symptomatic cohort (n=154) with radicular pain correlating to the level of a disc hernia.61 Takida et al. did a follow-up MRI every 3 months in a symptomatic cohort and found a similarly high regression rate for extrusions and sequestrations, but less so for protrusions.62 They also found a correlation between morphologic regression and a favorable clinical outcome, but their sample size was relatively small (n=42). Other studies have failed to correlate morphological development with clinical outcome. Various imaging characteristics predictive of spontaneous regression include contrast enhancement in the hernia tissue63 and high signal intensity on T2-weighted sequences.64 Interestingly, in a population-based cohort of middle-aged men and women that underwent MRI upon inclusion and again at 4- and 8-year follow-up, most hernias remained unchanged.65
When taking into account the available histological and immunohistochemical data discussed above, it thus seems that there is a correlation between spontaneous regression and inflammation (here defined as the presence of granulation tissue and inflammatory cells). The presence of inflammatory cells such as macrophages is often referred to in the literature as a mechanism of spontaneous regression. However, it can also be hypothesized that the inflammation causes swelling and formation of granulation tissue in a hernia and that the observed resorption is mainly a result of a decrease in this inflammatory reaction. Also, given the discrepancy in spontaneous regression rates in symptomatic cohorts compared to population-based cohorts it can be hypothesized that hernias can be categorized two ways: (1) active hernias, that are symptomatic and change in appearance over time due to an ongoing inflammatory reaction, and (2) inactive hernias, that neither cause pain nor change their morphological appearance over time due to the absence of an inflammatory reaction. Such inactive hernias may have previously been inflamed and/or symptomatic upon formation, causing acute lumbago and/or radiculopathy depending on its placement on the disc and proximity to nerve roots.
The morphology of herniated discs has received almost no attention in experimental research, likely due to challenges in developing representative animal models for the complex and heterogeneous development of a disc hernia. A clinical disc hernia most commonly occurs in a disc that is degenerated to some extent, and this two-step process is difficult to simulate in animal models. It has however been found that puncture of a lumbar intervertebral disc in the rat causes the formation of a hernia-like nodule over the puncture site, which forms over time.66 These nodules consist mainly of granulation tissue, and since a similar nodule can be found when placing autologous NP on a healthy disc, this granulation tissue likely forms as a reaction to components of the NP rather than the disc damage per se. Given the clinical data discussed above, this may constitute an interesting experimental model to study the granulation component of human disc hernias. However, neither treatment with TNF inhibitors nor methotrexate were found to affect the macroscopic appearance of the nodule in this model,67 indicating that the main mechanism behind the formation of the granulation tissue could either be the result of a reaction independent of the inflammatory process or that the treatment target lie upstream of the inflammatory cascade affected by the drugs.
To conclude, herniated discs consist not only of herniated disc material but also commonly of granulation tissue, and inflammatory cells are commonly observed in the herniated tissue. Spontaneous regression of disc hernias is common, but the correlation with clinical outcome remains controversial. There is a correlation between spontaneous regression and inflammation, but the causality between these is unknown. New experimental approaches to disc hernia morphology may constitute important methods to evaluate novel treatments.
INFECTION AS A CAUSE OF LUMBAR RADICULOPATHY
An alternative hypothesis that has received increasing attention during the last few years is the possibility of low-grade bacterial infection, primarily P. acnes, as a cause of inflammation in both discogenic low back pain and disc herniation. Stirling et al. found 53% of cultures of disc hernia tissue from patients undergoing surgery for disc herniation showed growth of bacteria, most commonly P. acnes.68 A relatively high frequency of positive tissue cultures, also most commonly P. acnes, from both herniated and degenerated, non-herniated discs have been subsequently reported in other studies as well.69-71 Albert et al. also found a correlation between positive cultures, post-operative Modic type 1 changes and chronic low back pain, and also reported that treatment with antibiotics was potent in reducing pain in a similar patient category.72 However, these results have not been reproduced, and as such the efficacy of antibiotics in chronic low back pain remains controversial and their use not recommended.
One of few studies on this subject also assessing contamination controls was published by Carricajo et al. They reported a low frequency (3.7%) of tissue cultures on herniated disc material positive for P. acnes, but many of the contamination control cultures obtained during surgery showed growth of P. acnes.73 A larger study with contamination controls aiming to elucidate the true role of low-virulent bacteria in disc degeneration and herniation is DISC (Degenerate-disc Infection Study with Contaminant Control), a large Australian cohort of patients undergoing spine surgery for various degenerative disc diseases, both cervical and lumbar. Preliminary data from 168 patients without contamination controls showed an overall positive culture frequency of 19.6%.74 Correlations were observed only between a positive culture and prior surgeries and having multilevel surgery, not between a positive culture and, for example, type of surgical procedure. These results do not support an infectious origin for the inflammatory process in disc herniation. Future results with contamination controls from larger studies such as DISC will hopefully allow for more certain conclusions to be drawn.
CONCLUSION
Lumbar radiculopathy is a complex and heterogeneous condition. Two distinct underlying mechanisms contribute to symptoms; pressure and inflammation. Pressure on the nerve root alone gives rise to nerve dysfunction symptoms, and circulatory compromise induced by the pressure is likely important for these symptoms. Inflammation causes nerve dysfunction symptoms as well, but also sensitizes the nerve root and makes it likely to produce pain when pressurized. Inflammation may also inherently induce pain in the absence of pressure as well. Nucleus pulposus triggers the inflammation in disc herniation, but the underlying mechanism is not well understood. Autoimmunity may be of importance, but it is not known to what extent. Low-virulent infections have been suggested as a cause of inflammation but is unlikely given current evidence. Also, disc hernias have been shown to be more dynamic structures than previously believed, with spontaneous regression as a commonly observed phenomenon. The development of new experimental approaches to disc herniation will be important to identifying and evaluating novel treatment options for lumbar radiculopathy.
SUGGESTED READING
- Chiu CC, Chuang TY, Chang KH, Wu CH, Lin PW, Hsu WY. The probability of spontaneous regression of lumbar herniated disc: a systematic review. Clin Rehabil. 2015;29(2):184-195.
- Di Martino A, Merlini L, Faldini C. Autoimmunity in intervertebral disc herniation: from bench to bedside. Expert Opin Ther Targets. 2013;17(12):1461-1470.
- Mulleman D, Mammou S, Griffoul I, Watier H, Goupille P. Pathophysiology of disk-related sciatica. I.--Evidence supporting a chemical component. Joint Bone Spine. 2006;73(2):151-158.
- Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44-56.
REFERENCES
- Truumees E. A history of lumbar disc herniation from Hippocrates to the 1990s. Clin Orthop Relat Res. 2015;473(6):1885-1895.
- Gelfan S, Tarlov IM. Physiology of spinal cord, nerve root and peripheral nerve compression. Am J Physiol. 1956;185(1):217-229.
- Sharpless S. Susceptibility of spinal nerve roots to compression block. The research status of spinal manipulative therapy. Goldstein M, ed NIH workshop Bethesda, MD: NINCDS Monograph. 1975:155-161.
- Olmarker K, Rydevik B, Holm S, Bagge U. Effects of experimental graded compression on blood flow in spinal nerve roots. A vital microscopic study on the porcine cauda equina. J Orthop Res. 1989;7(6):817-823.
- Olmarker K, Holm S, Rydevik B. Importance of compression onset rate for the degree of impairment of impulse propagation in experimental compression injury of the porcine cauda equina. Spine (Phila Pa 1976). 1990;15(5):416-419.
- Konno S, Yabuki S, Sato K, Olmarker K, Kikuchi S. A model for acute, chronic, and delayed graded compression of the dog cauda equina. Presentation of the gross, microscopic, and vascular anatomy of the dog cauda equina and accuracy in pressure transmission of the compression model. Spine (Phila Pa 1976). 1995;20(24):2758-2764.
- Sato K, Konno S, Yabuki S, Mao GP, Olmarker K, Kikuchi S. A model for acute, chronic, and delayed graded compression of the dog cauda equina. Neurophysiologic and histologic changes induced by acute, graded compression. Spine . 1995;20(22):2386-2391.
- Kikuchi S, Konno S, Kayama S, Sato K, Olmarker K. Increased resistance to acute compression injury in chronically compressed spinal nerve roots. An experimental study. Spine (Phila Pa 1976). 1996;21(22):2544-2550.
- Olmarker K, Rydevik B. Single- versus double-level nerve root compression. An experimental study on the porcine cauda equina with analyses of nerve impulse conduction properties. Clin Orthop Relat Res. 1992(279):35-39.
- Mao GP, Konno S, Arai I, Olmarker K, Kikuchi S. Chronic double-level cauda equina compression. An experimental study on the dog cauda equina with analyses of nerve conduction velocity. Spine (Phila Pa 1976). 1998;23(15):1641-1644.
- Rydevik B, Holm S, Brown MD, Lundborg G. Diffusion from the cerebrospinal fluid as a nutritional pathway for spinal nerve roots. Acta Physiol Scand. 1990;138(2):247-248.
- Otani K, Kikuchi S, Konno S, Olmarker K. Blood flow measurement in experimental chronic cauda equina compression in dogs: changes in blood flow at various conditions. J Spinal Disord. 2001;14(4):343-346.
- Takahashi K, Olmarker K, Holm S, Porter RW, Rydevik B. Double-level cauda equina compression: an experimental study with continuous monitoring of intraneural blood flow in the porcine cauda equina. J Orthop Res. 1993;11(1):104-109.
- Olmarker K, Rydevik B, Hansson T, Holm S. Compression-induced changes of the nutritional supply to the porcine cauda equina. J Spinal Disord. 1990;3(1):25-29.
- Olmarker K, Rydevik B, Holm S. Edema formation in spinal nerve roots induced by experimental, graded compression. An experimental study on the pig cauda equina with special reference to differences in effects between rapid and slow onset of compression. Spine (Phila Pa 1976). 1989;14(6):569-573.
- Kawakami M, Weinstein JN, Chatani K, Spratt KF, Meller ST, Gebhart GF. Experimental lumbar radiculopathy. Behavioral and histologic changes in a model of radicular pain after spinal nerve root irritation with chromic gut ligatures in the rat. Spine (Phila Pa 1976). 1994;19(16):1795-1802.
- Olmarker K, Storkson R, Berge OG. Pathogenesis of sciatic pain: a study of spontaneous behavior in rats exposed to experimental disc herniation. Spine (Phila Pa 1976). 2002;27(12):1312-1317.
- Kawakami M, Tamaki T, Hayashi N, et al. Mechanical compression of the lumbar nerve root alters pain-related behaviors induced by the nucleus pulposus in the rat. J Orthop Res. 2000;18(2):257-264.
- Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22(2):181-187.
- Yoshihara H. Prostaglandin E1 treatment for lumbar spinal canal stenosis: review of the literature. Pain Pract. 2016;16(2):245-256.
- Takahashi K, Shima I, Porter RW. Nerve root pressure in lumbar disc herniation. Spine (Phila Pa 1976). 1999;24(19):2003-2006.
- Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine (Phila Pa 1976). 1993;18(11):1425-1432.
- Olmarker K, Brisby H, Yabuki S, Nordborg C, Rydevik B. The effects of normal, frozen, and hyaluronidase-digested nucleus pulposus on nerve root structure and function. Spine (Phila Pa 1976). 1997;22(5):471-475; discussion 476.
- Olmarker K, Byrod G, Cornefjord M, Nordborg C, Rydevik B. Effects of methylprednisolone on nucleus pulposus-induced nerve root injury. Spine (Phila Pa 1976). 1994;19(16):1803-1808.
- Yabuki S, Kikuchi S, Olmarker K, Myers RR. Acute effects of nucleus pulposus on blood flow and endoneurial fluid pressure in rat dorsal root ganglia. Spine (Phila Pa 1976). 1998;23(23):2517-2523.
- Olmarker K, Blomquist J, Stromberg J, Nannmark U, Thomsen P, Rydevik B. Inflammatogenic properties of nucleus pulposus. Spine (Phila Pa 1976). 1995;20(6):665-669.
- Omarker K, Myers RR. Pathogenesis of sciatic pain: role of herniated nucleus pulposus and deformation of spinal nerve root and dorsal root ganglion. Pain. 1998;78(2):99-105.
- Shamji MF, Allen KD, So S, et al. Gait abnormalities and inflammatory cytokines in an autologous nucleus pulposus model of radiculopathy. Spine (Phila Pa 1976). 2009;34(7):648-654.
- Takebayashi T, Cavanaugh JM, Cuneyt Ozaktay A, Kallakuri S, Chen C. Effect of nucleus pulposus on the neural activity of dorsal root ganglion. Spine (Phila Pa 1976). 2001;26(8):940-945.
- Anzai H, Hamba M, Onda A, Konno S, Kikuchi S. Epidural application of nucleus pulposus enhances nociresponses of rat dorsal horn neurons. Spine (Phila Pa 1976). 2002;27(3):E50-55.
- Ohtori S, Inoue G, Koshi T, et al. Up-regulation of acid-sensing ion channel 3 in dorsal root ganglion neurons following application of nucleus pulposus on nerve root in rats. Spine (Phila Pa 1976). 2006;31(18):2048-2052.
- Yan J, Zou K, Liu X, et al. Hyperexcitability and sensitization of sodium channels of dorsal root ganglion neurons in a rat model of lumber disc herniation. Eur Spine J. 2016;25(1):177-185.
- Kato K, Sekiguchi M, Kikuchi S, Konno S. The effect of a 5-HT2A receptor antagonist on pain-related behavior, endogenous 5-hydroxytryptamine production, and the expression 5-HT2A receptors in dorsal root ganglia in a rat lumbar disc herniation model. Spine (Phila Pa 1976). 2015;40(6):357-362.
- Kanayama M, Hashimoto T, Shigenobu K, Oha F, Yamane S. New treatment of lumbar disc herniation involving 5-hydroxytryptamine2A receptor inhibitor: a randomized controlled trial. J Neurosurg Spine. 2005;2(4):441-446.
- Saito H, Wakai J, Sekiguchi M, Kikuchi S, Konno S. The effect of selective serotonin reuptake inhibitor (SSRI) on pain-related behavior in a rat model of neuropathic pain. Eur Spine J. 2014;23(11):2401-2409.
- Byrod G, Otani K, Brisby H, Rydevik B, Olmarker K. Methylprednisolone reduces the early vascular permeability increase in spinal nerve roots induced by epidural nucleus pulposus application. J Orthop Res. 2000;18(6):983-987.
- Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine (Phila Pa 1976). 1998;23(23):2538-2544.
- Cuellar JM, Montesano PX, Carstens E. Role of TNF-alpha in sensitization of nociceptive dorsal horn neurons induced by application of nucleus pulposus to L5 dorsal root ganglion in rats. Pain. 2004;110(3):578-587.
- Murata Y, Onda A, Rydevik B, Takahashi K, Olmarker K. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced histologic changes in the dorsal root ganglion. Spine (Phila Pa 1976). 2004;29(22):2477-2484.
- Murata Y, Nannmark U, Rydevik B, Takahashi K, Olmarker K. The role of tumor necrosis factor-alpha in apoptosis of dorsal root ganglion cells induced by herniated nucleus pulposus in rats. Spine(Phila Pa 1976). 2008;33(2):155-162.
- Kobayashi H, Kikuchi S, Konno S, Kato K, Sekiguchi M. Interaction of 5-hydroxytryptamine and tumor necrosis factor-a to pain-related behavior by nucleus pulposus applied on the nerve root in rats. Spine (Phila Pa 1976). 2011;36(3):210-218.
- Jonsson D, Finskas O, Fujioka Y, Stahlberg A, Olmarker K. Experimental disc herniation in the rat causes downregulation of serotonin receptor 2c in a TNF-dependent manner. Clin Orthop Relat Res. 2015;473(6):1913-1919.
- Aoki Y, Rydevik B, Kikuchi S, Olmarker K. Local application of disc-related cytokines on spinal nerve roots. Spine (Phila Pa 1976). 2002;27(15):1614-1617.
- Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine (Phila Pa 1976). 2001;26(8):863-869.
- Onda A, Yabuki S, Kikuchi S. Effects of neutralizing antibodies to tumor necrosis factor-alpha on nucleus pulposus-induced abnormal nociresponses in rat dorsal horn neurons. Spine (Phila Pa 1976). 2003;28(10):967-972.
- Olmarker K, Nutu M, Storkson R. Changes in spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective TNF-alpha inhibition. Spine (Phila Pa 1976). 2003;28(15):1635-1641; discussion 1642.
- de Souza Grava AL, Ferrari LF, Defino HL. Cytokine inhibition and time-related influence of inflammatory stimuli on the hyperalgesia induced by the nucleus pulposus. Eur Spine J. 2012;21(3):537-545.
- Olmarker K. Combination of two cytokine inhibitors reduces nucleus pulposus-induced nerve injury more than using each inhibitor separately. Open Orthop J. 2011;5:151-153.
- Cuellar JM, Borges PM, Cuellar VG, Yoo A, Scuderi GJ, Yeomans DC. Cytokine expression in the epidural space: a model of noncompressive disc herniation-induced inflammation. Spine (Phila Pa 1976). 2013;38(1):17-23.
- Murata Y, Rydevik B, Nannmark U, et al. Local application of interleukin-6 to the dorsal root ganglion induces tumor necrosis factor-a in the dorsal root ganglion and results in apoptosis of the dorsal root ganglion cells. Spine (Phila Pa 1976). 2011;36(12):926-932.
- Pennington JB, McCarron RF, Laros GS. Identification of IgG in the canine intervertebral disc. Spine (Phila Pa 1976). 1988;13(8):909-912.
- Satoh K, Konno S, Nishiyama K, Olmarker K, Kikuchi S. Presence and distribution of antigen-antibody complexes in the herniated nucleus pulposus. Spine (Phila Pa 1976). 1999;24(19):1980-1984.
- Capossela S, Schlafli P, Bertolo A, et al. Degenerated human intervertebral discs contain autoantibodies against extracellular matrix proteins. Eur Cell Mater. 2014;27:251-263; discussion 263.
- Geiss A, Larsson K, Junevik K, Rydevik B, Olmarker K. Autologous nucleus pulposus primes T cells to develop into interleukin-4-producing effector cells: an experimental study on the autoimmune properties of nucleus pulposus. J Orthop Res. 2009;27(1):97-103.
- Geiss A, Larsson K, Rydevik B, Takahashi I, Olmarker K. Autoimmune properties of nucleus pulposus: an experimental study in pigs. Spine (Phila Pa 1976). 2007;32(2):168-173.
- Wang YF, Chen PY, Chang W, et al. Clinical significance of tumor necrosis factor-a inhibitors in the treatment of sciatica: a systematic review and meta-analysis. PLoS One. 2014;9(7):e103147.
- Kokubo Y, Uchida K, Kobayashi S, et al. Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J Neurosurg Spine. 2008;9(3):285-295.
- Winkler D, Hammer N, Gossner J, Schober R, Vitzthum HE, Meixensberger J. Does histology predict the clinical outcome after lumbar intervertebral disc herniation: no. Med Hypotheses. 2013;80(3):215-219.
- Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF 3rd, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976). 1996;21(3):271-277.
- Yasuma T, Arai K, Yamauchi Y. The histology of lumbar intervertebral disc herniation. The significance of small blood vessels in the extruded tissue. Spine (Phila Pa 1976). 1993;18(13):1761-1765.
- Jensen TS, Albert HB, Soerensen JS, Manniche C, Leboeuf-Yde C. Natural course of disc morphology in patients with sciatica: an MRI study using a standardized qualitative classification system. Spine (Phila Pa 1976). 2006;31(14):1605-1612; discussion 1613.
- Takada E, Takahashi M, Shimada K. Natural history of lumbar disc hernia with radicular leg pain: spontaneous MRI changes of the herniated mass and correlation with clinical outcome. J Orthop Surg (Hong Kong). 2001;9(1):1-7.
- Splendiani A, Puglielli E, De Amicis R, Barile A, Masciocchi C, Gallucci M. Spontaneous resolution of lumbar disk herniation: predictive signs for prognostic evaluation. Neuroradiology. 2004;46(11):916-922.
- Henmi T, Sairyo K, Nakano S, et al. Natural history of extruded lumbar intervertebral disc herniation. J Med Invest. 2002;49(1-2):40-43.
- Kjaer P, Tunset A, Boyle E, Jensen TS. Progression of lumbar disc herniations over an eight-year period in a group of adult Danes from the general population--a longitudinal MRI study using quantitative measures. BMC Musculoskelet Disord. 2016;17:26.
- Olmarker K. Puncture of a disc and application of nucleus pulposus induces disc herniation-like changes and osteophytes. An experimental study in rats. Open Orthop J. 2011;5:154-159.
- Olmarker K. Formation of nucleus pulposus-induced disc hernia-like nodules on the disc surface is not induced by TNF. The Internet Journal of Spine Surgery. 2009;5(2):1-5.
- Stirling A, Worthington T, Rafiq M, Lambert PA, Elliott TS. Association between sciatica and Propionibacterium acnes. Lancet. 2001;357(9273):2024-2025.
- Agarwal V, Golish SR, Alamin TF. Bacteriologic culture of excised intervertebral disc from immunocompetent patients undergoing single level primary lumbar microdiscectomy. J Spinal Disord Tech. 2011;24(6):397-400.
- Albert HB, Lambert P, Rollason J, et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J. 2013;22(4):690-696.
- Arndt J, Charles YP, Koebel C, Bogorin I, Steib JP. Bacteriology of degenerated lumbar intervertebral disks. J Spinal Disord Tech. 2012;25(7):E211-216.
- Albert HB, Sorensen JS, Christensen BS, Manniche C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J. 2013;22(4):697-707.
- Carricajo A, Nuti C, Aubert E, et al. Propionibacterium acnes contamination in lumbar disc surgery. J Hosp Infect. 2007;66(3):275-277.
- Rao PJ, Phan K, Reddy R, Scherman DB, Taylor P, Mobbs RJ. DISC (degenerate-disc infection study with contaminant control): pilot study of australian cohort of patients without the contaminant control. Spine (Phila Pa 1976). 2016;41(11):935-939.