Nicolas Plais, MD and Federico P. Girardi, MD
INTRODUCTION/CONCEPTS
In 2003,1 Bertagnoli et al. described the transpsoas approach to access the retroperitoneal lumbar spine. In 2006,2 Ozgur et al. described a minimally invasive technique for anterior lumbar interbody fusion with less morbidity than the classic retroperitoneal approach. Today, lateral lumbar interbody fusion (LLIF) is a well-known minimally invasive surgery (MIS) technique that permits anterior column interbody fusion via a direct lateral transpsoas approach. This technique is becoming increasingly popular among spinal surgeons and is featured in the spine literature. Since its inception more than 12 years ago, indications for LLIF have been expanded from the management of degenerative spine disease to spinal deformities. The objective of this chapter is to review the advantages and drawbacks of the LLIF technique, indications and surgical technique. In well-indicated patients, LLIF can be an effective technique to address spinal diseases in a safe and minimally invasive way.
MAIN ADVANTAGES OF LLIF
The advantages of the technique can be classified according to three main features:
- Approach
- Biomechanics
- Minimally Invasive Surgery
Approach
LLIF is a lateral, retroperitoneal approach that avoids the great vessels and decreases the risk of vascular injury. Given the relatively low vascular injury risk, the use of an access surgeon is usually unnecessary.
Biomechanics
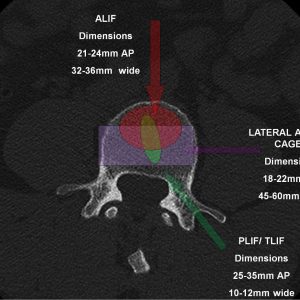
Compared to posterior lumbar interbody fusion (PLIF) or transforaminal lumbar interbody fusion (TLIF) techniques, LLIF allows the use of larger cages that span the apophyseal ring of the vertebra (Fig. 5-1). The cortical bone at the periphery of the vertebral body is stronger than the inner end plates. With the larger available footprint for LLIF, biomechanically, there is more stability and a lower rate of subsidence.3 Moreover, LLIF preserves the anterior longitudinal ligament (ALL), which is one of the major stabilizing forces in spine extension as well as the posterior longitudinal ligament (PLL). The retention of these spinal ligaments provides extra support for the cage and allows standalone constructs.
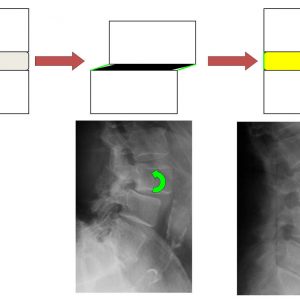
Through the use of wider and taller cages than those permitted in other approaches like PLIF or TLIF, LLIF allows indirect central and foraminal decompression. In fact, when the height of the disk is restored, the foramen opens and widens. According to Kepler et al., the average foraminal area can increase up to 35%.4 Moreover, the preservation of both the ALL and PLL provides an efficient reduction of vertebral bodies through ligamentotaxis, decreasing central stenosis5 (Fig 5-2). LLIF has also been shown to be an effective technique to correct coronal deformity in adult spinal deformity.6
Minimally Invasive Surgery
Many studies2-7 have reported low blood loss, shorter hospital stay and recovery, and minor muscle dissection and postoperative muscle atrophy for patients who underwent LLIF.
Indications and contraindications
Given the anatomy of the lumbar plexus, there are some concerns about the risk of nerve injury with a lateral approach. In a systematic review, Lehmen et al. reported evidence that supports LLIF as a safe surgical treatment at all levels of the lumbar spine and in particular at the L4-L5 level.8 However, the authors emphasize the importance of patient selection. In recent years, there is increasing interest in the LLIF technique and various authors have reported the feasibility and outcomes of LLIF for a wide array of indications, such as:
- Symptomatic degenerative disc disease,
- Total disc replacement,9
- Foraminal stenosis,4
- Lumbar spinal stenosis,10
- Degenerative spondylolisthesis,
- Non-union,
- Adjacent disease,11
- Trauma,
- Degenerative scoliosis (standalone or combined with a posterior surgery),6-12
- Revision surgery and removal of implants (e.g., cages, total disk replacement), and
- A new approach for motion preservation device implantation.9
Absolute contraindications
The LLIF technique presents some limitations, and its contraindications must be considered. First, this approach is not appropriate for surgeries involving the L5-S1 segment. The presence of the iliac crest prohibits lateral access. A pure anterior, oblique or posterior surgical approach must be chosen as an alternative for this level. A rising psoas sign13 is related to a high risk of femoral nerve injuries and aborted surgeries. The presence of this sign on preoperative MRI contraindicates the LLIF technique.
Other contraindications for consideration
Osteoporosis or previous abdominal surgeries are relative contraindications to LLIF technique as the risks associated with the approach or for cage subsidence increase significantly. Moreover, patients with any evidence of fusion (especially posterior locked facets) are not considered good candidates for indirect decompression. Attempted distraction of a fused level will likely result in endplate damage and a high risk of subsidence. In these cases, a posterior approach would be more optimal.
Finally, many authors have reported that the LLIF technique does not improve regional lumbar lordosis or global sagittal alignment in adult spine deformities with fixed and rigid curves or with sagittal malalignment (sagittal vertical axis superior to 10 cm).14
Lateral Anatomy of the Lumbar Spine
Understanding regional anatomy is integral to performing a safe LLIF procedure. The structures present in the retroperitoneal space (a virtual anatomic space which lies between the parietal peritoneum and the abdominal wall) include the psoas muscle, lumbar plexus, great vessels (aorta, vena cava and common iliacs), kidney, ureters and adrenal glands. The relation of these structures and the implication on the surgical technique are detailed below.
The Lumbar Plexus
The lumbar plexus is a complex neural network formed by the ventral branches of the first to the fourth upper lumbar nerves (L1-L4). It has seven major branches that pass through the psoas major and run obliquely down through the pelvis. The relation of the branches with the psoas muscle has been thoroughly studied. As Benglis stressed, the intrapsoas part of the plexus may be at high risk during transpsoas dissection.15 The visualization of the roots is also difficult and constitutes a barrier for lateral surgeries. A description of the roots and the risk presented during the surgery is detailed below.
The last thoracic nerve (or T12) usually supplements the first lumbar level (L1) to form the iliohypogastric nerve and the ilioinguinal nerve. The iliohypogastric nerve supplies sensation to the buttock and hypogastric regions and the ilioinguinal nerve supplies sensation to the groin and external genitalia. These two sensory branches may be injured during the blunt dissection of the oblique muscles.
L2, L3 and L4 form the lateral femoral cutaneous nerve, femoral nerve and the obturator nerve. The femoral nerve supplies the hip flexors and knee extensors while the obturator nerve supplies the adductor muscles of the leg. These branches are at risk during dissection of the psoas. A direct injury or an indirect lesion (by distraction or by ischemia) to the femoral nerve is extremely debilitating and can lead to difficulty walking.
In 2010, Uribe et al. defined16 the safe working zones for the transpsoas approach through a cadaveric study. The authors divided the vertebral body in four quartiles in the sagittal plane and demonstrated that the safe zones, understood as the absence of crossing of a lumbar plexus branch, were always anterior to zone IV and were different for every level. The risk of neural injury increases at the lowest levels and is the greatest at the L4-L5 segment. At this level, the psoas muscle is more expansive and the femoral nerve more ventral, making a femoral nerve injury or a lumbosacral plexopathy more likely.
Special attention should also be paid to the genitofemoral nerve. This nerve is formed by the union of axons of the L1 and L2 roots and supply sensation to the upper anterior thigh as well as the skin of the anterior scrotum in males and mons pubis in females. The genitofemoral nerve is the only nerve found ventral to zone III.
The ventral rami of the fourth and fifth lumbar nerve of the lumbosacral trunk, descend to join the sacral plexus.
Great Vessels
As described by Deukmejian et al.,17 the abdominal aorta and the inferior cava vein structures move anterior to the surgical corridor when the patient is positioned in the lateral decubitus position. For this reason, a low rate of vascular injuries have been reported. However, an anterior migration of the instruments or the retractor can produce an injury to the great vessels or to the segmental vessels.
Peritoneum
To develop the retroperitoneal plane, the peritoneum needs to be mobilized anteriorly. If incompletely developed because of previous scar tissue or inappropriate technique, the peritoneum and the underlying structures can be injured.
Description of the Technique
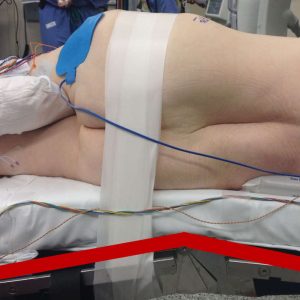
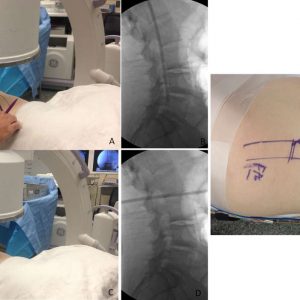
The patient is positioned in the right lateral decubitus with the knees flexed. All pressure points must be padded well and an axillary roll placed to protect the brachial plexus. Taping is used at the axilla and pelvis to stabilize the patient’s shoulder and pelvis to ensure landmarks obtained during fluoroscopy are not moved during surgery (Fig. 5-3).
When the L4-L5 disk space is involved, coronal angulation of this level must be corrected. The patient should be positioned in lateral hyperflexion to expose this level correctly.
Once the patient is positioned, x-rays are performed. Anterior-posterior and lateral views without rotation must be obtained to localize the surgical level and draw the skin landmarks for the incision. Matching the lordotic angle to the C-arm ensures optimal cage insertion and height assessment.
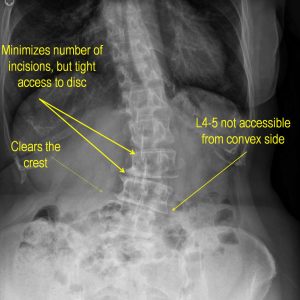
In the absence of a coronal curve, the incision is made on the symptomatic side of the patient (Fig. 5-4).
In patients with a coronal deformity, however, the location of the incision is placed at the concavity of the curve, which allows treatment of a greater number of levels with a significantly smaller incision (Fig. 5-5).
Intraoperative neuromonitoring and fluoroscopic guidance are mandatory during this procedure. As stated by Lehmen et al.,8 clear evidence supports the use of advanced neuromonitoring techniques. Appropriate monitoring devices and especially electromyography and somatosensory evoked potentials (SSEPs) are advocated. Moreover, Silverstein et al.18 described that the monitoring of the saphenous nerve with SSEPs is useful to detect femoral nerve injury during the approach. After positioning and incision planning has been performed, a review of the operative consent form, surgical checklists and a proper time out is customary.
Surgical Approach
Two techniques have been described:
Through a tubular dilator—this technique requires the successive insertion of tubular dilators. Since no direct visualization is possible, directional probing and root mapping with direct nerve stimulation (EMG) is required.
Mini-Open—this technique is the author’s preferred technique. Direct visualization of the roots is associated with directional probing, making the technique safer.
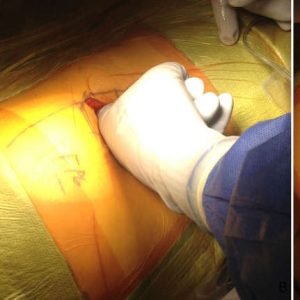
For a one or two level fusion, a small oblique incision with a surgical #10 blade scalpel is centered on the level previously marked on the skin. When more than 2 levels are involved, a second incision may be useful. Hemostasis with the Bovie electrocautery is immediately accomplished. Following, a blunt dissection with a muscle-splitting approach is performed. The external oblique, internal oblique and transverse abdominis muscles are individually split along the direction of their respective fibers. This allows access to the retroperitoneal space, which is entered and developed under direct vision. The visceral sac is gently rotated medially where the psoas musculature can be clearly identified (Fig. 5-6).
As explained before, dissection of the psoas must be realized with neuro-surveillance. A handheld nerve detection apparatus is used to localize the position of the exiting nerve roots at each level. Care must be taken to maintain a dissection plane ventral to the neural elements throughout the course of the operative procedure. The psoas fibers are gently split along the direction of their long axis, thus exposing the underlying disk space.
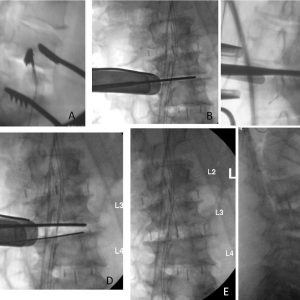
Once the disk level has been confirmed by fluoroscopy, a self-retaining retractor system with light magnification is placed to maintain exposure (Fig. 5-7).

If a different level must be operated on, a new dissection of the psoas must be performed. Exposure of the new disk space can be accomplished by gentle splitting of psoas fibers along the direction of their long axis.
Disk preparation
An annulotomy with a surgical #10 blade scalpel is performed followed by the discectomy. A pituitary rongeur is used first to remove the disk material, and gentle distraction is applied with Cobb elevators. All endplate cartilage must be carefully removed. Appropriate sizing for implant placement must then be performed utilizing bullet distractors in sequence of increasing size. The sizes of the trials vary from 8, 10 and 12 mm. It is important to penetrate the contralateral side. Irrigation is advised before implant positioning. Position of the trial is confirmed using biplanar fluoroscopy (Fig. 5-8).
Bone grafting
Many alternatives exist for bone grafting. The implant may be filled with a biologic allograft such as bone morphogenetic protein-2 (BMP-2) or iliac crest autograft. Once the graft material is introduced, its position must be checked again on both AP and lateral views.
Closure
Prior to closure, meticulous hemostasis must be achieved. Once the preliminary sponge, clamp and needle counts are correct, oblique musculature of the flank is closed in layers using a #1 Vicryl suture placed in an interrupted figure eight fashion. Scarpa’s fascia and subcutaneous tissues are reapproximated using 2-0 Vicryl suture placed in an interrupted fashion with the knots buried. Lastly, skin edges are reapproximated using a 3-0 Monocryl suture placed in continuous subcuticular fashion. Sterile dressings are then applied.
Outcomes
As highlighted by Lehmen et al.,8 more than 43 studies have published outcomes of patients treated with the LLIF technique. Several high quality publications19,20 support favorable long term outcomes.
Kotwal et al.19 reported, for a cohort of 141 consecutive patients treated with mini-invasive LLIF, pain improved 53% compared to baseline, disability measured by the Oswestry Disability Index (ODI) improved by 43%, and quality of life physical component summary (PCS) improved by 41%. In patients with coronal deformity, lumbar Cobb angle decreased from an average of 24.8° to 13.6°. Isaacs et al.20 present similar results with 87.3% satisfaction rates, significant disc height increase, and reduction of listhesis with high fusion rates.
However, as underlined by Lehmen et al.,8 there is a lack of consistency in the results due to the heterogeneity of the treatments and patients’ characteristics. Long-term follow-up and higher-level evidence are still necessary to determine the effectiveness of LLIF compared to conventional surgical approaches.
Complications
Tohmeh et al.21 report the results from a prospective, multi-center study after LLIF at L3-L4 and/or L4-L5 have shown an overall perioperative complication rate of 5%. Complications can be divided in short-term (approach related) and long-term. The following are the short-term complications:
Hip flexion weakness—hip flexion weakness is very common postoperatively and has been considered a side effect of the technique. Tohmeh21 reports a rate as high as 27.5%, and it is thought to be related to the trauma caused to the psoas muscle. It is generally transient and resolves in the first two weeks after surgery.
Nerve injury—Seddon’s classification divides nerve injury into neurapraxia, axonotmesis or neurotmesis. Lumbar neurapraxia is by far the most frequent with rates up to 62.7% that have been reported.4
Anterior thigh sensory deficit—a sensory deficit of the anterior thigh presents a different etiology and may be caused by an excessively aggressive retraction. In a study of 919 treated levels, Lykissas et al.22 reported rates of 38.0% sensory deficits which typically resolve in the first two weeks postoperatively.
Motor deficits—a motor deficit rate of 2.9 to 4.6%8 has been reported and can be caused by two different mechanisms. Indirect injuries are related to excessive retraction time (≥ 30 minutes) and/or extension of the psoas muscle opening. The retraction produces an ischemic injury to the nerve. On the other hand, direct injuries are much less frequent and occur during the approach. Femoral nerve or obturator nerve injuries are the most feared complication. Nerves are formed by different roots and a nerve lesion is clinically much more significant than an injury of a unique root, which can happen during a TLIF procedure. Motor deficits, however, are recoverable, and the severity of the neurologic deficit decreases over time.22,23 The time for symptom resolution is about 6 months. At long-term follow-up with a minimum of 12 months, up to 2.9% of surgery-related motor deficits persist.23 The outcome and recovery time of these motor deficit injuries are much less predictable than other types of complications. Motor deficits can lead to a persistent disability.
Autonomic injuries—autonomic injuries, including those of the lumbar sympathetic trunk, have been described. These types of injuries may lead to dysfunction of lower viscera, lower extremity and sex organs.
Vascular injury (L4-L5)—vascular injuries are uncommon in the LLIF approach. Uribe et al.24 reported 0.10% incidence of vascular injury, which is significantly lower than the vascular complications reported in ALIF procedures. Brau25 et al. reported rates of 0.8% for arterial injuries and 0.8% for venous injuries in 750 cases of pure anterior approach. Although rare, injuries to the iliac or great vessels can be fatal. In 2013, Aichmair et al.11 reported a case of aortic perforation requiring immediate laparotomy during a LLIF procedure.
Other short-term complications—end-plate fracture during cage insertion, urological injury, incisional hernia, bowel injury with Olgivie Syndrome (0-12%), or infection have also been reported.8
Long-term complications include the following:
Implant subsidence—cage migration is infrequent but has been described.26 Lateral migration is associated with leg pain and can occur even with pedicle screws. In cases of end-plate breaches and cage subsidence into the vertebral body, the foramen can close and the effective decompression could be lost. The use of wider implants decreases this potential complication. Le et al.27 reported a rate of 2% of clinical subsidence and 14.3% of radiographic subsidence. Considerations for prevention of implant subsidence include avoiding over distraction, protecting the endplates and treating osteoporosis preoperatively. Moreover, the use of BMP or posterior instrumentation can be also utilized to reduce the risk of subsidence.
Rate of revision surgery—as described by Nemani et al.,10 the rate of revision surgery in standalone technique has been reported to be as high as 10.3%. Causes for revision are multiple and involve new radiculopathy, subsidence, coronal and/or sagittal decompensation, lumbar stenosis, or pseudarthrosis.
DISCUSSION/CONCLUSION
The limitations of LLIF include the inability to treat the L5-S1 segment by this approach, increased neurological risk at the L4-L5 segment and challenges when accessing the disc space in severe coronal deformities to avoid injury. Moreover, surgeons and the operating room staff are exposed to radiation during the procedure and the technique presents its own anatomical and approach complication risk profile. The risk of nerve injury is a major concern and electromyography or EMG monitoring is unable to prevent sensory deficits, which are by far, the most frequent injuries.
However, LLIF is a technique that can be effective for a variety of indications. LLIF allows a thorough discectomy from a lateral annulotomy. The approach utilizes wider cages and thereby larger footprints with a lower rate of pseudarthrosis and cage migration. Additionally, LLIF can be used in the treatment of a wide range of degenerative spinal diseases. Recently, the approach has been extended as a possible strategy for non-fusion surgery, revision fusion and salvage surgery.
In addition, multiple levels can be treated with a small incision while preserving the abdominal muscles. The lateral approach has comparable clinical outcomes and fewer complications compared to other approaches.
The restoration of disc height provides an efficient decompression of the neural elements. The major effect of the LLIF decompression is obtained on opening of the foramen and decompressing the lateral recess. Central canal stenosis is also improved with the LLIF technique but not with the same effectiveness.
LLIF is also a useful technique for deformity correction. Many authors have shown that LLIF is effective in correcting coronal plane alignment.6,7 The technique can be used in a standalone procedure, especially in older patients, because of the lower morbidity, or combined with a supplemental internal device from the same approach (lateral plating) or a different approach (pedicle screws). However, as its effect on restoring lordosis is limited, LLIF is suboptimal for the correction of sagittal deformities.
LLIF is a safe and reproducible technique. Its role in the treatment of spinal diseases will continue to be elucidated in the next years. A comprehensive understanding of the technique, the regional anatomy and its complications is critical to performing lateral fusion in a safe way for patients.
REFERENCES
- Bertagnoli R, Vazquez RJ. The Anterolateral TransPsoatic Approach (ALPA): a new technique for implanting prosthetic disc-nucleus devices. J Spinal Disord Tech. 2003;16(4):398-404.
- Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435-443.
- Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine. 2013;19(1):110-118.
- Kepler CK, Sharma AK, Huang RC, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine. 2012;16(4):329-333.
- Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976). 2010;35(26 Suppl):S331-S337.
- Castro C, Oliveira L, Amaral R, Marchi L, Pimenta L. Is the lateral transpsoas approach feasible for the treatment of adult degenerative scoliosis? Clin Orthop Relat Res. 2014;472(6):1776-1783.
- Ahmadian A, Bach K, Bolinger B, et al. Stand-alone minimally invasive lateral lumbar interbody fusion: multicenter clinical outcomes. J Clin Neurosci. 2015;22(4):740-746.
- Lehmen JA, Gerber EJ. MIS lateral spine surgery: a systematic literature review of complications, outcomes, and economics. Eur Spine J. 2015;24 Suppl 3:287-313.
- Pimenta L, Oliveira L, Schaffa T, Coutinho E, Marchi L. Lumbar total disc replacement from an extreme lateral approach: clinical experience with a minimum of 2 years’ follow-up. J Neurosurg Spine. 2011;14(1):38-45.
- Nemani VM, Aichmair A, Taher F, et al. Rate of revision surgery after stand-alone lateral lumbar interbody fusion for lumbar spinal stenosis. Spine (Phila Pa 1976). 2014;39(5):E326-331.
- Aichmair A, Alimi M, Hughes AP, et al. Single-level lateral lumbar interbody fusion for the treatment of adjacent segment disease: a retrospective two-center study. Spine J. 2014;14(11):S158-S159.
- Mummaneni PV, Shaffrey CI, Lenke LG, et al. The minimally invasive spinal deformity surgery algorithm: a reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg Focus. 2014;36(5):E6.
- Voyadzis JM, Felbaum D, Rhee J. The rising psoas sign: an analysis of preoperative imaging characteristics of aborted minimally invasive lateral interbody fusions at L4-5. J Neurosurg Spine. 2014;20(5):531-537.
- Acosta FL, Liu J, Slimack N, Moller D, Fessler R, Koski T. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults: a radiographic study. J Neurosurg Spine. 2011;15(1):92-96.
- Benglis DM, Vanni S, Levi AD. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10(2):139-144.
- Uribe JS, Arredondo N, Dakwar E, Vale FL. Defining the safe working zones using the minimally invasive lateral retroperitoneal transpsoas approach: an anatomical study. J Neurosurg Spine. 2010;13(2):260-266.
- Deukmedjian AR, Le TV, Dakwar E, Martinez CR, Uribe JS. Movement of abdominal structures on magnetic resonance imaging during positioning changes related to lateral lumbar spine surgery: a morphometric study: clinical article. J Neurosurg Spine. 2012;16(6):615-623.
- Silverstein J, Mermelstein L, DeWal H, Basra S. Saphenous nerve somatosensory evoked potentials: a novel technique to monitor the femoral nerve during transpsoas lumbar lateral interbody fusion. Spine (Phila Pa 1976). 2014;39(15):1254-1260.
- Kotwal S, Kawaguchi S, Lebl D, et al. Minimally invasive lateral lumbar interbody fusion: clinical and radiographic outcome at a minimum 2-year follow-up. J Spinal Disord Tech. 2015;28(4):119-125.
- Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976). 2010;35(26 Suppl):S322-S330.
- Tohmeh AG, Rodgers WB, Peterson MD. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine. 2011;14(1):31-37.
- Lykissas MG, Aichmair A, Hughes AP, et al. Nerve injury after lateral lumbar interbody fusion: a review of 919 treated levels with identification of risk factors. Spine J. 2014;14(5):749-758.
- Pumberger M, Hughes AP, Huang RR, Sama AA, Cammisa FP, Girardi FP. Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J. 2012;21(6):1192-1199.
- Uribe JS, Deukmedjian AR. Visceral, vascular, and wound complications following over 13,000 lateral interbody fusions: a survey study and literature review. Eur Spine J. 2015;24 Suppl 3:386-396.
- Brau SA. Mini-open approach to the spine for anterior lumbar interbody fusion: description of the procedure, results and complications. Spine J. 2002;2(3):216-223.
- Daffner SD, Wang JC. Migrated XLIF cage: case report and discussion of surgical technique. Orthopedics. 2010;33(7):518.
- Le TV, Baaj AA., Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976). 2012;37(14):1268-1273.