Steven Bokshan, Roy Ruttiman, Adam E.M. Eltorai, and Alan H. Daniels
INTRODUCTION
Parkinson’s disease, Parkinson’s related disease, poliomyelitis and cerebral palsy are causes of adult spinal deformity in the United States. In addition to causing spinal deformity, these conditions may also exacerbate existing spinal deformity. While the true etiology of spinal deformity in these conditions remains incompletely understood, it is most likely attributable to multiple, interrelated factors. Surgical management is multifaceted and requires a patient-specific approach that considers the patient’s functional status and responsiveness to medication. Furthermore, the relationship between the spinal deformity and the patient’s symptomatology must be carefully assessed before committing to surgical intervention. Successful spinal deformity surgeries are possible through optimized pre- and postoperative care, familiarity with unique challenges during surgery, meticulous surgical execution and careful patient selection.
Parkinson’s Disease and Parkinson’s Related Disease
Pathophysiology, etiology and spinal deformity
Parkinson’s disease (PD) and its related diseases (e.g., Multiple Systems Atrophy) are degenerative movement disorders characterized by tremor, rigidity and akinesia. Patients with these disorders often develop postural changes including stooped posture, dropped head, and a flexed trunk, hips and knees.1,2 Unfortunately, these postural changes often manifest as more disabling spinal deformities such as scoliosis, antecollis, camptocormia and Pisa syndrome. Abnormalities of spinal alignment shift the patient’s center of gravity, forcing abnormal spinal load-bearing, which in turn may accelerate normal and age-related spinal degeneration.3 These biomechanical and neurophysiological changes place PD patients at increased risk for spinal deformity.4
The underlying cause of spinal disorders in PD remains controversial.3 Possible contributing factors include muscle rigidity, proprioceptive disintegration, medication effects and soft tissue alteration.3 Dystonia and myopathy have also been explored as causal origins of spinal deformity. The dystonia theory asserts that postural disorders—particularly the characteristic stooped posture—arise from abnormal flexion of the paraspinal and truncal muscles.3,5 Such abnormal muscular contractions have been clinically observed in patients with PD presenting with early antecollis and camptocormia.5
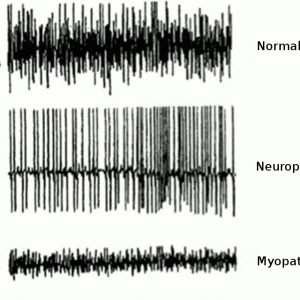
The involvement of dystonia may be suggested by electromyography (EMG) studies, although these studies are often inconsistent.6 For example, Di Matteo et al. found characteristic dystonic activity in the paraspinal muscles of just three of ten patients with PD presenting with Pisa syndrome (lateral spinal tilt).6
EMG studies (Fig. 5-1) on truncal muscles in patients with PD have revealed myopathic patterns (i.e., fibrillation potentials and small polyphasic motor unit potentials).7 Similarly, computed tomography (CT) studies in patients with PD frequently demonstrate muscle atrophy restricted to paraspinal musculature.5 Histological studies have shown myopathic changes including ragged red fibers, myofibrillar disorganization, nuclear polymorphism, cellular atrophy and proliferation in extra-muscular connective tissue, although these findings may sometimes also be found in age-matched controls without Parkinson’s disease.5 With progressive PD, typical dystonic features more commonly seen in early onset Parkinson’s are progressively hallmarked by focal myopathy, characterized by pronounced muscular changes and spinal deformity.
Select cases may shed light on the pathophysiology of PD associated spinal deformity. Uzawa et al. reported cases in which dopamine agonists caused or worsened Pisa syndrome or antecollis in PD patients within weeks to months of treatment, both effects subsequently correcting upon discontinuing the medications.8 Neurotransmitter imbalances secondary to medications may dysregulate axial muscle tone, contributing to spinal changes.8 However, most cases of PD-associated spinal deformity are not affected by medication.
Soft-tissue changes are a potential pathophysiological mechanism in the development of axial skeletal deformities. Ashour et al. described alterations in soft-tissue, particularly connective tissue fibrosis and muscle atrophy and subsequent fixed contracture development, as the possible underlying mechanism of limb deformities seen in PD patients, which may also contribute to spinal deformity.7 Further research is needed to assess whether such soft tissue changes may also be at the root of axial skeletal deformities.
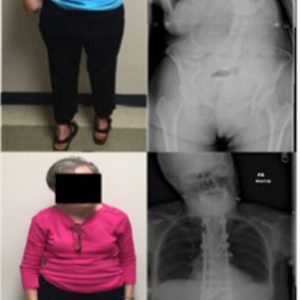
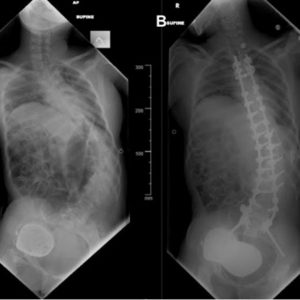
Scoliosis may occur in patients with Parkinson’s disease and refers to a coronal curvature of the spine, frequently coupled with vertebral rotation (Fig. 5-2).3 Parkinson’s disease scoliosis is often associated with a loss of lordosis in the setting of lateral listhesis and spondylolisthesis.3 Patients with PD are more likely to develop scoliosis than the general elderly population.3 The prevalence of scoliosis in patients with PD ranges from 8.5-60% as compared to 6-32% in healthy age-matched individuals.3
Antecollis is a forward flexion of the head and neck—commonly referred to as dropped head syndrome—coupled with increased axial tone (Fig. 5-3). As the deformity progresses, patients with antecollis display markedly reduced range of motion and eventually develop a fixed deformity.9 Antecollis is most common in patients with PD or peripheral neuromuscular disorder such as multiple system atrophy.2 Approximately 6% of PD patients develop antecollis. There is mixed evidence supporting use of levodopa, clonazepam, botulinum toxin injections and deep brain stimulation to assist in the treatment of antecollis in PD.3
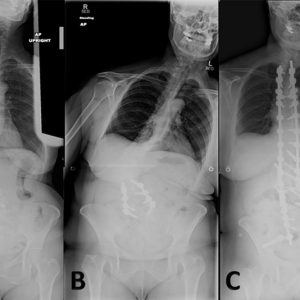
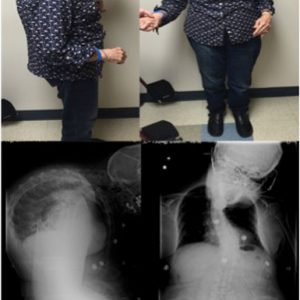
Although studies evaluating vertebral fusion to treat antecollis are limited, surgical treatment may benefit individuals with poor enteral intake or difficulties breathing.1,9 Furthermore, weakened upper back muscles and extensor muscles of the neck seen in antecollis may increase the risk of instrumentation failure at the lower instrumented vertebra; therefore, many surgeons advocate extending the fusion to the low thoracic or upper lumbar spine.5 Spinal osteotomy may be required if the deformity is fixed.
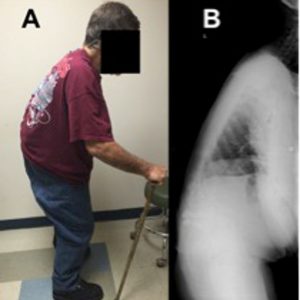
Camptocormia is described as pronounced forward flexion of the thoracolumbar spine, typically characterized by positive sagittal balance and reduced lumbar lordosis when standing (Fig. 5-4).7 Early camptocormia often corrects when the inflicted patient is supine, although this deformity may become fixed when long-standing. The prevalence of camptocormia in PD patients is between 3% and 18%.3,7 Camptocormia typically presents 5-10 years after the onset of PD, affects older patients and is associated with more severe PD.3
Various treatment strategies have yielded equivocal but often poor results for camptocormia in PD patients—including orthoses, levodopa, botulinum toxin injections and deep brain stimulation.2,3
Spinal surgery is a beneficial treatment option for camptocormia.2,3 Optimization of post-operative sagittal balance is mandatory to prevent proximal junctional kyphosis.1,3 Many patients with PD-associated camptocormia have kyphotic failure at the cephalad junction of the deformity and eventually undergo upper cervical or even occiput to ilium fusions.1
Pisa syndrome is characterized by more than 15° of lateral truncal flexion when sitting or standing, which often resolves with passive mobilization or supine positioning (Fig. 5-5).3 Affecting 2% of PD patients, Pisa syndrome has been described as a truncal dystonia, a cholinergic-dopaminergic imbalance, and as a possible precursor to scoliosis in PD.3 As the deformity evolves, patients are prone to falling and may experience pain from rib-pelvis abutment.
Dopaminergic therapy is effective in some cases as a treatment option in early stage Pisa syndrome.8 Anticholinergics, botulinum toxin injections and deep brain stimulation treatment strategies have demonstrated mixed results.8 Like scoliosis, Pisa syndrome patients with severe coronal plane deformity or concomitant myelopathy or radiculopathy may benefit from long-segment spinal arthrodesis with correction of the deformity.1-3
Operative considerations
Treatment of spinal deformity in patients with neuromuscular disease is focused on decreasing pain and preserving function by optimizing spinal alignment. While the type of surgical intervention performed is highly patient-specific, emphasis must be placed on spino-pelvic balance often achieved with rigid fixation usually from the upper thoracic spine to the pelvis.10
The management of spine deformities in patients with PD requires careful consideration, surgical planning and instrumentation with robust spinal fixation due to common mechanical instrumentation failure and junctional kyphosis. Patients with PD commonly possess decreased bone mineral density, weakened paraspinal and truncal muscular strength and concomitant medical problems.10 Pre-operatively, parkinsonian symptoms should be carefully managed with medication or deep brain stimulation, and bone mineral density should be optimized with appropriate calcium and vitamin D supplementation along with weight-bearing exercise. If spine surgery is indicated, preoperative assessment must account for the biomechanical challenges associated with PD.10 The patient’s functional level, medication regimen, life goals and natural progression of PD should all inform the development of a patient-specific treatment plan. While medication, activity modification and physiotherapy may serve as viable treatment options for most patients with PD, surgery may be recommended for patients with refractory disease.1,2,10
As with spinal deformities due to other neuromuscular diseases, surgical treatment of spinal deformity in PD differs from that of degenerative and idiopathic scoliosis. Spinal deformity in PD may exhibit characteristic fixed lumbar sagittal and coronal deformity with little associated rotational deformity, as opposed to idiopathic scoliosis, which is characterized by axial rotational deformity.1-3 In PD patients, prevention of spinal deformity progression and preservation of spino-pelvic balance may be achieved by spinal fusion from the upper thoracic spine to the pelvis, with an emphasis on reestablishing physiological lumbar lordosis and proper global sagittal alignment.1,2,10
The creation of a strong fusion construct is essential in patients with neuromuscular spinal deformity, as these deformities are often rigid and in need of significant correction with long segmented constructs. Furthermore, with some studies showing an incidence of rod fracture of up to 22%, outrigger support with additional rods should be considered in order to increase the stiffness of the construct; this is particularly true when osteotomies are performed.11 Finally, the fusion construct must also be optimized with meticulous decortication, the use of appropriately contoured rods, and the appropriate usage of graft and biologic materials.11
Surgical outcomes
PD patients with spinal deformity are at risk of intra- and post-operative complications, with many patents requiring revision surgery.1,10 Post-operative infection (17%) and instrumentation failure and/or screw pull-out (35%) accounted for the majority of surgical complications in a study performed by Koller et al., with 33% of all patients necessitating revision surgery.10 Complications were linked to pseudarthrosis and junctional kyphosis, while surgical success was associated with restoration of sagittal balance.10
Some series have demonstrated very poor outcomes following treatment of PD associated spinal deformity. In a 14-patient study by Babat et al., 86% (12 patients) of PD patients who underwent spinal surgery required additional surgery, with the majority of patients (76%) requiring surgeries at the same or adjacent level to address instability.1 Two patients (14%) experienced wound infections during the course of treatment. Babat et al. recognized kyphosis or segmental instability at the operative site or adjacent levels as the principle mechanism of failure.1
Despite some poor outcomes, Koller et al. found that the majority of patients with PD (78%) who underwent spinal surgery for spinal deformities were satisfied with their clinical outcomes, whereas only 22% of patients were either not satisfied or uncertain of their outcomes.10
The number of days in hospital care for patients with PD is greater than for the general population who underwent the same spinal procedures.3 Several factors may contribute to poor outcomes in PD patients including delayed mobility, longer hospitalization time, reduced mobility, and other comorbidities. As such, close monitoring of potential complications and early initiation of rehabilitation is even more important in PD patients than it is in the general population.3
Poliomyelitis
Pathophysiology, etiology and spinal deformity
Poliomyelitis is an acute viral infection caused by the poliovirus, preferentially targeting the anterior horn cells of the central nervous system within the spinal cord.12 Following acute viral infection, nearly 30% of patients may develop flaccid limb paralysis and subsequent post-poliomyelitis paralytic scoliosis.12 Even with the advent of an effective vaccine, chronic poliomyelitis remains the most prevalent motor neuron disease in the United States due to high prevalence prior to widespread vaccination, with approximately 1.6 million patients living with poliomyelitis in the United States and nearly 20 million worldwide.13,14 Polio has been eradicated in most of the developed world, but still occurs in Pakistan and Afghanistan as an endemic disease and other developing countries as a vaccine-derived illness.
While a paucity of data exists describing the pathophysiology of post-poliomyelitis paralytic scoliosis, most hypotheses are centered around asymmetric involvement of anterior horn cells leading to a subsequent asymmetric muscular loading on the axial spine.14,15 The progressive loss of anterior horn cells is thought to be a result of increased metabolism from the viral infection or through an autoimmune-mediated process by which the immune system targets affected neurons.12 Asymmetric paralytic involvement of the abdominal wall muscles and the quadratus lumborum consistently produce the common scoliotic patterns seen in post-polio paralytic scoliosis.15
While progressive scoliosis is a common result of progressive poliomyelitis, these patients are also at risk for developing lower extremity contractures, limb length discrepancies and increases in pelvic obliquity as a result of trunk-muscle imbalance.16 Classically, these fixed paralytic deformities are described as an affected hip held in abduction, a contralateral hip adduction deformity with superior pelvic elevation and a scoliotic curve with a convexity directed towards the affected side; however, variations of these are commonly encountered.16
The classic deformity experienced in post-poliomyelitis scoliosis is a long, right-sided “C”-curve including the cervical, thoracic and lumbar spine, although many more recent case series do not support this dogma.15 In their series of 118 patients, Mayer et al. found that the most common deformities were double structural thoracic and lumbar curves (78%) followed by double structural thoracic and thoracolumbar curves (17%), with only 4 instances of the long “C” curve.15 The average pre-operative pelvic obliquity was 22.7 degrees.15
As polio is a chronic disease, many patients ultimately go on to develop progressive paralysis and worsening scoliosis resistant to non-operative management.15 In the terminal stages of disease, patients become non-ambulatory and surgical intervention is indicated to provide a stable torso over a level pelvis.15 In this regard, surgical intervention reduces the risk of ischial pressure sores, increases self-image and allows patients to use their hands in activities of daily living.15
Operative considerations
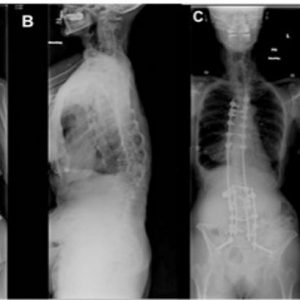
Surgical correction of spinal deformity seen in post-polio scoliosis is aimed at preservation of spino-pelvic alignment (Fig. 5-6). While little has been published regarding operative management of post-polio scoliosis, Mayer et al. reported a curve correction of 49% using a staged anterior fusion Dywer technique followed by a posterior fusion with Harrington rods.15 They concluded that an anterior fusion to L5 commonly achieved the majority of the correction on, and should be recommended for, more severe curves prior to posterior surgical fusion.15 With the advent of newer pedicle screw fixation techniques, Godzik et al. show a correction rate of 33% at 2 year follow-up with 50% of patients undergoing a combined anterior and posterior approach and the other 50% undergoing a posterior approach.17 These authors also report a clinically significant improvement in the SRS-22 domains of satisfaction, pain and self-image following surgical intervention.17
Surgical outcomes
Patients undergoing spinal deformity correction for post-polio scoliosis appear to have a higher rate of complications, approaching 45% in some series, or nearly triple that observed in routine scoliosis surgery.15,17 In particular, the rate of a new neurological deficit following correction surgery ranges from 5-18%, although some of these deficits may be transient.17 Of note, intraoperative neurological monitoring has been shown to have a 13% false negative rate, requiring a careful neurological assessment following the sugery.17 These iatrogenic injuries may be accounted for by the complexity of deformity correction involved in post-polio scoliosis, particularly in cases where multiple osteotomies are required. Other complications that are common to post-polio syndrome include development of pseudarthrosis (1.8-40%), increased blood loss, instrumentation failure and post-operative infection rates as high as 20%.17 More recent accounts report infection rates much lower than this, which may reflect evolving surgical technique.15,17
Cerebral Palsy
Pathophysiology, etiology and spinal deformity
Cerebral palsy (CP) represents a non-progressive encephalopathy caused by brain injury during the pre-, peri- or post-natal period and may be characterized by lack of voluntary muscle control, increased muscle spasticity, decreased muscle tone, variable seizure disorders and varying degrees of mental impairment.18 CP occurs in as many as 5 per 1000 live births with a nearly 21% to 76% incidence rate of scoliosis.18 Patients with CP may be non-ambulatory and successful treatment often relies on the establishment of an appropriate sitting balance in order to facilitate transfers and skin care.18
The deformities and gait imbalances seen in cerebral palsy are a result of prolonged muscle weakness and spasticity, with approximately 90% of the muscle tone abnormalities being spastically induced.19 In patients with CP, incoming sensory signals from the afferent nervous system are unable to be downregulated by the inadequately functioning central nervous system, leading to spastic reflex arcs and inefficient muscular co-contractions which ultimately lead to muscle weakness.19 Downregulation of the overactive afferent nervous system has become the key to medical and surgical management of spasticity and forms the basis of intrathecal baclofen pumps and selective dorsal rhizotomy surgery.19
Special care must be taken with a multidisciplinary team in order to appropriately treat the numerous comorbidities experienced with CP including increased risk of aspiration and pneumonia, gastroesophageal disorders such as dysphagia and gastric reflux (occurs in 15%–75% of patients), malnutrition (46%–90%) and infection risk.18 If a seizure disorder is present, history of valproic acid usage should be ascertained as this may increase intraoperative blood loss.18 Similar to poliomyelitis, CP is frequently accompanied by pelvic obliquity and hip deformity that must be evaluated prior to any intervention. Particularly in patients with spastic quadriplegia, contractures of the hip adductors and flexors may lead to increased pelvic obliquity and an increased risk of hip dislocations.18 A targeted physical and radiographic examination should be performed in order to differentiate suprapubic, infrapubic or mixed causes of pelvic tilt with subsequent intervention targeting the cause. For example, the examiner may place the patient prone, with his or her legs dangling over the side of the examination table. If the pelvic obliquity reduces with the legs off the side of the table, this suggests that the pelvic obliquity is a result of the pelvic-femoral musculature.18 On the contrary, if the pelvic obliquity persists in the setting of extension and adduction/abduction of the legs, this is more likely a result of a fixed spino-pelvic obliquity.18
Adults with CP may have a number of different curve patterns depending on the severity of the disease. Lonstein et al. classify these deformities into the two most commonly seen categories; group 1 curves are single thoracic or double thoracic/lumbar curves with a level pelvis (commonly seen in ambulatory patients) while group 2 curves are long thoracolumbar or C-shaped curves with associated pelvic obliquity (frequently seen in non-ambulatory patients).20 Commonly, adults with CP have curves that are generally stiffer than their younger counterparts. The natural curve progression of CP usually involves a flexible curve arising between 3 and 10 years old with a rapid progression to a rigid curve thereafter.20 While treatment often consists of orthotics and wedges to promote an appropriate sitting balance, surgical intervention is usually indicated for curves greater than 40 degrees, loss of sitting balance and significant curve progression (most commonly seen during the adolescent growth spurt).20
Operative considerations
The surgical management of patients with cerebral palsy is complex and must be tailored to each individual patient. Surgery is generally indicated for curves greater than 40 degrees, loss of sitting balance and significant curve progression (Fig. 5-7).20 With regard to the proximal extent of fusion, patients with CP often suffer from upper thoracic kyphosis coupled with poor head control, often necessitating fusion to T3 or higher.20 For ambulatory patients without significant pelvic obliquity (less than 15 degrees), an effort should be made to stop the fusion at L4 or L5, in an effort to spare the motion that comes with the lumbosacral junction.20 In ambulatory patients with a structural lumbosacral curve, fusion to the pelvis may be required, although this must be done sparingly given its effect on gait.20 While there are no standard accepted criteria for performing an anterior release, some authors advocate for an anterior release with stiff curves greater than 70 degrees, although the need for anterior release is increasingly being challenged with the advent of newer pedicle screw fixation and the correction that may be achieved with osteotomies.20
Surgical outcomes
The complication rate following scoliosis surgery for individuals with cerebral palsy ranges from 44% to 80%, with as high as a 7% perioperative death rate.20 In particular, patients with CP are at risk for post-operative pulmonary complications (aspiration, respiratory failure), gastroesophageal hemorrhage or reflux, and infection.20 More specifically, children with CP have a 24% rate of pulmonary infection, an 18% rate of urinary tract infection (UTI), and a 5% rate of wound infection, with adults likely suffering equal or higher rates.20 As a result, multidisciplinary care is required post-operatively with special attention directed towards positioning in effort to avoid aspiration. Routine use of anti-reflux medication should strongly be considered.
Ultimately, CP patients undergoing deformity correction experience improvements in pain, happiness, frequency of feeling sick and tired, and parental satisfaction by one year postoperatively, although there is little data to support an improvement in physical function.21
Additional Perioperative Considerations
Given the propensity for patients with neuromuscular disease to develop perioperative complications, it is essential that a multidisciplinary team of operating room staff, anesthesiologists, nurses, therapists and family participate in appropriate perioperative care.20,21 Preoperatively, patient symptoms should be carefully managed with medications or deep brain stimulation in the case of PD or with baclofen or dorsal rhizotomy surgery in the case of CP.14 It is essential that surgeons are familiar with the patients’ medication regimen, as these medications may increase the risk of intraoperative blood loss, a risk that is already elevated compared to baseline in patients with neuromuscular disease.22 Surgeons should be familiar with baclofen pumps in order to manage these devices during the surgical approach and to interrogate an appropriately functioning device to prevent post-operative baclofen withdrawal. Indeed, baclofen withdrawal can be fatal if not recognized in a timely manner and often manifests as acute changes in mental status, confusion, fevers and severe spasms. Finally, direct alimentary delivery of Parkinson’s medications through an infusion-regulated nasogastric tube is increasingly being adopted in an effort to prevent perioperative disease exacerbations, thus requiring that surgeons are familiar with the infusion rates of these medications.
Pulmonary complications are common in patients with neuromuscular disease. For example, Bendon et al. found that nearly one quarter of patients with CP undergoing spinal deformity correction ultimately required ventilation following surgery as a result of a post-operative respiratory complications.23 Preoperative assessment of underlying respiratory disease should be performed. Likewise, it is imperative that anesthesia staff be familiar with the complexities of intraoperative positioning and ventilation requirements. Ideally, these staff members should have specialized training in cases with large spinal deformity correction.
Postoperative wound infection is of significant concern, as patients with neuromuscular diseases are often at increased rates of postoperative infection.21-23 Many surgeons advocate for the usage of intrasite, lyophilized vancomycin power applied directly to the wound, as several studies have showed a decreased incidence of surgical site infection following both cervical and thoracolumbar instrumented fusion, although these findings remain controversial.24 To date, these studies are largely retrospective in nature and additional randomized controlled trials are needed with the consideration of the addition of intrasite vancomycin for neuromuscular patients.
Perioperative IV broad-spectrum prophylactic antibiosis is strongly encouraged, as patients with neuromuscular disease are commonly found to have gram negative surgical site infections that are not encountered in routine orthopedic surgery. Finally, the usage of negative pressure wound therapy has grown significantly over the last decade and should be considered following deformity correction in cases where additional boundary protection is required.25 Patients should be placed in an intensive care unit familiar with the specialized needs of these patients. Mobilization and frequent turns are required to prevent wound breakdown and ischial pressure ulcers. Likewise, physical therapy should also begin immediately following surgery in order to work on gait training and core muscle strengthening, as these interventions work synergistically with deformity correction surgery.
CONCLUSION
As the medical management of individuals with neuromuscular disease improves, so too will the future rate of adult spinal deformity, particularly as these individuals live to older ages. In addition to causing spinal deformity, neuromuscular conditions may also exacerbate existing spinal deformity. While the true etiology of spinal deformity from neuromuscular disease remains incompletely understood, it is most likely attributable to multiple interrelated factors. Surgical management is multifaceted and requires a patient-specific approach that considers the patient’s functional status and responsiveness to medication. It is essential that a multidisciplinary team of operating room staff, anesthesiologists, nurses, therapists and family participate in appropriate perioperative care for these individuals. Management of these deformities requires attention to detail to minimize the risk of perioperative complications and maximize quality of life.
REFERENCES
- Babat LB, McLain RF, Bingaman W, Kalfas I, Young P, Rufo-Smith C. Spinal surgery in patients with Parkinson’s disease: construct failure and progressive deformity. Spine. 2004;29(18):2006-2012.
- Moon SH, Lee HM, Chun HJ, et al. Surgical outcome of lumbar fusion surgery in patients with Parkinson disease. J Spinal Disord Tech. 2012;25(7):351-355.
- Ha Y, Oh JK, Smith JS, et al. Impact of movement disorders on management of spinal deformity in the elderly. Neurosurgery. 2015;77 Suppl 4:S173-S185.
- van de Warrenburg BP, Cordivari C, Ryan AM, et al. The phenomenon of disproportionate antecollis in Parkinson’s disease and multiple system atrophy.Mov Disord. 2007;22(16):2325-2331.
- Peeraully T, Tan EK. Camptocormia in Parkinson’s disease: dystonia or myopathy? Basal Ganglia. 2012;2(1):1-3.
- Di Matteo A, Fasano A, Squintani G, et al. Lateral trunk flexion in Parkinson’s disease: EMG features disclose two different underlying pathophysiological mechanisms. J Neurol. 2011;258(5):740-745.
- Ashour R, Jankovic J. Joint and skeletal deformities in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Mov Disord. 2006;21(11):1856-1863.
- Uzawa A, Mori M, Kojima S, et al. Dopamine agonist‐induced antecollis in Parkinson’s disease. Mov Disord. 2009;24(16):2408-2411.
- Jorens P, Eycken M, Parizel G, Martin JJ. Antecollis in parkinsonism. Lancet. 1989;1(8650):1320-1321.
- Koller H, Acosta F, Zenner J, et al. Spinal surgery in patients with Parkinson’s disease: experiences with the challenges posed by sagittal imbalance and the Parkinson’s spine. Eur Spine J. 2010;19(10):1785-1794.
- Palumbo MA, Shah KN, Eberson CP, Hart RA, Daniels AH. Outrigger rod technique for supplemental support of posterior spinal arthrodesis. Spine J. 2015;15(6):1409-1414.
- Dalakas MC, Elder G, Hallett M, et al. A long-term follow-up study of patients with post-poliomyelitis neuromuscular symptoms. N Engl J Med. 1986;314(15):959-963.
- Gonzalez H, Olsson T, Borg K. Management of postpolio syndrome. Lancet Neurol. 2010;9(6):634-642.
- Jubelt B, Agre JC. Characteristics and management of postpolio syndrome. JAMA. 2000;284(4):412-414.
- Mayer PJ, Dove J, Ditmanson M, Shen YS. Post-poliomyelitis paralytic scoliosis. A review of curve patterns and results of surgical treatments in 118 consecutive patients. Spine (Phila Pa 1976). 1981;6(6):573-582.
- Lee DY, Choi IH, Chung CY, Cho TJ, Lee JC. Fixed pelvic obliquity after poliomyelitis: classification and management. J Bone Joint Surg Br. 1997;79(2):190-196.
- Godzik J, Lenke LG, Holekamp T, Sides B, Kelly MP. Complications and outcomes of complex spine reconstructions in poliomyelitis-associated spinal deformities: a single-institution experience. Spine (Phila Pa 1976). 2014;39(15):1211-1216.
- Jones-Quaidoo SM, Yang S, Arlet V. Surgical management of spinal deformities in cerebral palsy. A review. J Neurosurg Spine. 2010;13(6):672-685.
- Krägeloh-Mann I, Horber V. The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: a systematic review. Dev Med Child Neurol. 2007;49(2):144-151.
- Lonstein JE, Akbarnia BA. Operative treatment of spinal deformities in patients with cerebral palsy or mental retardation. An analysis of one hundred and seven cases. J Bone Joint Surg Am. 1983;65(1):43-55.
- Jones KB, Sponseller PD, Shindle MK, McCarthy ML. Longitudinal parental perceptions of spinal fusion for neuromuscular spine deformity in patients with totally involved cerebral palsy. J Pediatr Orthop. 2003;23(2):143-149.
- Chambers HG, Weinstein CH, Mubarak SJ, Wenger DR, Silva PD. The effect of valproic acid on blood loss in patients with cerebral palsy. J Pediatr Orthop. 1999;19(6):792-795.
- Bendon AA, George KA, Patel D. Perioperative complications and outcomes in children with cerebral palsy undergoing scoliosis surgery. Paediatr Anaesth. 2016 Oct 1;26(10):970-975.
- Martin JR, Adogwa O, Brown CR, et al. Experience with intrawound vancomycin powder for spinal deformity surgery. Spine (Phila Pa 1976). 2014;39(2):177-184.
- Mehbod AA, Ogilvie JW, Pinto MR, et al. Postoperative deep wound infections in adults after spinal fusion: management with vacuum-assisted wound closure. J Spinal Disord Tech. 2005;18(1):14-17.