Brian L. Hollenbeck and Kevin J. McGuire
INTRODUCTION
Measurement of patient outcomes after surgical intervention has become an integral part of assessing hospital quality and is vital to patient’s ability to make an informed decision about spine surgery. Recent advances in outcome measurements have been made possible by the availability of large-scale databases from patient registries, electronic medical records and insurance billing information. In this chapter, we discuss the role of risk adjustment when measuring patient outcomes, with particular emphasis on use of the Surgical Invasiveness Index (SII) to account for procedural variation.
Patient outcome measurements are used for multiple purposes, including furthering epidemiologic understanding of procedural risk and identifying variation in surgeon performance. Organizations utilize this information for calculating financial liability associated with bundled payments or other non-traditional payment structures. Insurers also use this information to assess quality and value of providers and organizations. As patient outcome measurements become more available, so too has the variation in quality become more evident.1 As an example, utilization of spine surgery varies across different geographic locations2 and type of hospital,3 and acceptance of adverse events is not consistent.4
In this era of heightened focus on patient outcomes, there is an equal focus on methodology to adjust risk appropriately. In fields as heterogeneous as spine surgery, risk adjustment is a necessary tool to fairly compare “apples to apples” and avoid the “apples to oranges” comparisons that yield inaccurate and misleading information. In this context, it is helpful to think of patient outcomes as being dependent upon (a) patient risk factors, (b) risk inherent to the procedure, (c) variation among providers or organizations, and (d) random variation,5,6 as illustrated in the following equation:
Outcome = Patient Factors + Procedural Factors + Surgeon/Organization Variation + Random Variation
One must be cognizant of the piece of this equation that one is measuring. For instance, if an insurer wishes to assess variation in patient outcomes that are attributable to an organization and/or surgeon, then it is necessary to adjust for the “patient factors” and “procedural factors” that may confound the “surgeon/organization variation” measurement that is desired. In this chapter, we will discuss the role that the SII plays in risk adjustment models, with particular focus on using this index as a global measurement of “procedural factors.”
Ideal Risk Adjustment Variables
Ideal variables used for risk adjustment should be biologically plausible; they should be consistent when measured by different organizations or practices, and they should be independent of other measured variables (they should not be collinear). Below, we will use the example of risk factors for surgical site infection to illustrate when risk adjustment is beneficial and how the above ideals are not always applicable to risk adjustment in the real world.
Surgical Site Infection
Surgical site infection is an excellent example of the strengths and limitations of outcome measurement in spine surgery. Numerous studies have identified risk factors associated with surgical site infection, and knowledge of these risk factors has helped to inform patient selection for surgery and quality initiatives for reducing infection after spine surgery.3,6-10
Risk factors for surgical site infection after spine surgery are well described (Table 3-1). This list focuses only on variables that have been identified as significant in studies that used multivariable regression models for statistical analysis.3,9,11-25 When the list is expanded to include risk factors seen in univariate analyses, then the following putative risk factors are also included: smoking status, bowel incontinence, previous history of spine surgery, length of preoperative hospitalization, “complexity” of the surgery, multilevel surgery, hematoma formation, seroma formation, dural leak, perioperative steroid injection, increased blood loss, immunosuppression, degree of intraoperative tissue damage, and infection at remote body sites.
| Variable | Variable Classification | References |
|---|---|---|
| Morbid Obesity | Patient Factor | 11,13,15,16,17 |
| Age ≥ 65 | Patient Factor | 14,15,18 |
| ASA Score | Patient Factor | 3,9 |
| Diabetes | Patient Factor | 3,13,16 |
| Urinary incontinence | Patient Factor | 17 |
| Nutritional Status | Patient Factor | 18 |
| Gender | Patient Factor | 18 |
| Ethnicity | Patient Factor | 13 |
| Chronic Steroid Use | Patient Factor | 19 |
| Hypertension | Patient Factor | 11 |
| Hemato-oncological morbidity | Patient Factor | 19 |
| Renal Disease | Patient Factor | 11,19 |
| Smoking Status | Patient Factor | 20 |
| Prior Spine Infection | Patient Factor | 16 |
| Staphylococcus aureus colonization | Patient Factor | 21 |
| Trauma | Patient and Procedural | 15,22 |
| Surgery Duration | Patient, Procedural, or Institutional | 3,14,19,23 |
| Level of surgery | Procedural Factor | 3,11,12,13,18 |
| Use of Instrumentation | Procedural Factor | 18,24 |
| Surgical Approach | Procedural Factor | 3,12,17,20,24,25 |
| Surgical Invasiveness Index | Procedural Factor | 11,15 |
| Intraoperative Blood Loss | Procedural or Institutional Factor | 20 |
| Iodine surgical preparation | Institutional Factor | 23 |
| Preoperative delay > 1 hour | Institutional Factor | 25 |
| Medical School Affiliation | Institutional Factor | 3 |
| Hospital Bed Size | Institutional Factor | 3 |
Fortunately, infections after spine surgery have become uncommon—occurring in 1-4% of cases in recent series.3,11,12 This success, however, creates challenges when attempting to measure risk- adjusted patient outcomes. Using multivariable regression models for risk adjustment, one would require a database with approximately 300 SSI cases and (assuming a 1% infection rate) approximately 30,000 controls to achieve adequate power for calculations. This degree of power is not always attainable. A practical way around this power limitation is development of composite scores to comprise the entirety or near-entirety of a risk category.
In learning about risk adjustment, surgery duration is an illustrative example of another problem that is commonly encountered. Suppose one is performing a risk-adjusted patient outcome measurement to compare infection rates among surgeons in a single institution. Surgery duration may be included in the risk adjustment model, as this has frequently been identified as a predictor of increased infection risk in spine surgery.13,14 However, surgery duration in this setting is a surrogate marker for a variety of other biologically plausible variables. For instance, surgery may take two hours longer in a patient who is morbidly obese and has a history of prior surgery. In this case, it would be preferable to adjust for BMI and history of prior surgery (“patient factors”) rather than duration, as these are more indicative of the underlying biologic process that is increasing risk. Surgery duration also depends on the procedure being performed, and in this case, it would be optimal to adjust for the type of surgical procedure rather than surgery duration. In both of the above examples, the risk that is portrayed by surgery duration is indicative of patient risk or procedural risk and should be accounted for in order to best assess variation between surgeons.
In contrast, there may be times when surgery duration is a part of the variation that we want to measure, and we therefore do not want to adjust for it. For instance, if a surgeon who does mostly cervical laminectomy attempts a complex staged anterior/posterior fusion of the lumbar spine and sacrum, the procedure will likely take longer than it would for a surgeon who performs this procedure regularly. In this case, the increased risk portrayed by surgery duration is a direct measurement of surgeon variability that is in question. It would be wrong to adjust for surgery duration in this case, as it will skew the outcome measurement towards the norm.
A real-world solution to the problems presented above is to identify risk scores that encompass a variety of patient or procedural risk factors and can be used as single variables during risk adjustment. Below, we will discuss how SII accurately encompasses the “procedural” risk associated with spine surgery.
INVASIVENESS INDEX
Invasiveness Index Measurement
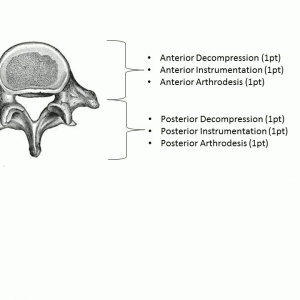
To calculate Invasiveness Index, one point is assigned for each of the following six interventions at each spinal level involved in the procedure: anterior decompression, anterior arthrodesis, anterior instrumentation, posterior decompression, posterior arthrodesis, and posterior instrumentation (Table 3-2). The midline of the spinal cord is used as the designation between anterior and posterior. Surgical approach is not considered into the score. One level is defined as a vertebral body and the corresponding disc that is caudal to the vertebral body (Fig. 3-1).
| Component | Definition |
|---|---|
| Anterior Decompression | 1 unit for each vertebra requiring partial or complete excision of the vertebral body or the disc caudal to that vertebra |
| Anterior Fusion | 1 unit for each vertebra that has graft material attached to or replacing that vertebral body |
| Anterior Instrumentation | 1 unit for each vertebra body that has screws, plate, cage, or structural graft attached to its vertebral body or replacing its vertebral body |
| Posterior Decompression | 1 unit for each vertebra requiring laminectomy or foraminotomy at the foramen caudal to its pedicle and/or discectomy at the disc caudal to that vertebral body |
| Posterior Fusion | 1 unit for each vertebra that has graft material on its lamina, facets or transverse processes |
| Posterior Instrumentation | 1 unit for each vertebra that has screws, hooks, or wires attached to its pedicles, facets, lamina or transverse processes |
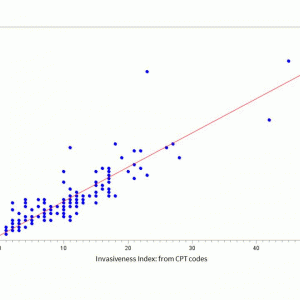
A L2-L5 laminectomy with instrumented spinal fusion of L4-5 using local autograft and allograft from a posterior approach would be assigned four points for posterior decompression, two points for L4-L5 posterior instrumentation, and two points for L4-L5 posterior arthrodesis. The SII score for this procedure is eight. A C4-C7 anterior cervical discectomy and fusion is assigned four points for anterior decompression and four points for anterior fusion. This procedure also has an SII score of eight. A posterolateral L3-L5 laminectomy with L3-L4 arthrodesis and interbody cage at L3-L4 would be assigned four points for posterior decompression, two points for posterior arthrodesis, two points for posterior instrumentation, two points for anterior decompression and two points for anterior instrumentation, for a total SII score of twelve. For additional examples of invasiveness index, refer to Mirza et al.6,26 or Cizik et al.11 SII can be calculated manually or through CPT codes, as has been previously validated15,27(Fig. 3-2).
Invasiveness Index as a Predictor of Patient Outcomes
Mirza et al. first described the Surgical Invasiveness Index in 2006 as a tool to adjust for procedural variation when assessing safety of spine surgery.6 In this study, 210 spine surgery cases from three surgeons (one orthopedic, two neurosurgeons) were monitored prospectively with active surveillance for adverse events. A total of 176 adverse events were defined and validated by a multi-disciplinary team of orthopedic surgeons, neurosurgeons, hospitalists, anesthesiologists, nurses and operating room managers. SII was found to be consistent when measured by researchers or clinicians.
The same University of Washington group subsequently validated the SII with a prospective cohort study consisting of 1,723 spine surgery patients from 17 surgeons (8 orthopaedic, 9 neurosurgery) in two tertiary-care hospitals.26 The researchers found that SII was strongly associated with other markers of surgical invasiveness and poor outcomes. SII accounted for 44% of the variation seen in blood loss, and 52% of the variation seen in surgery duration. Other variables (comprised of spine region, revision surgery, Charleston Comorbidity Score, age, gender, body mass index, spine region, diagnosis, and neurologic status) accounted for only 1–5% each of the variation seen in both blood loss and surgery duration.
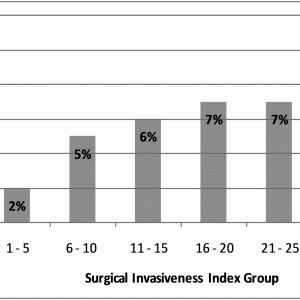
A subsequent publication using the same patient cohort demonstrated that SII is strongly associated with surgical site infection. In this study, surgical site infection was defined as a return to the operating room for debridement and irrigation due to infection; infections that did not require a return to the operating room were excluded. Patients with SII of 1–5 demonstrated a 2% risk of infection, whereas patients with SII > 25 demonstrated an 11% risk of infection. This relationship was upheld in multivariable regression, where only SII, spine level, renal disease, hypertension, and BMI were significant predictors of infection (Fig. 3-3). Of these, SII conferred the highest relative risk in multivariable analysis (using SII 1–5 as the reference group: SII 6–10 relative risk (RR) 2.24 (1.21 – 3.86), SII >20 RR 3.15 (1.37 – 6.99)).
SII has also been assessed as a risk factor for infection using standard definitions set by the Center for Disease Control and the National Healthcare Safety Network (NHSN), and in this cohort, SII also performed well as a predictor of surgical site infection.15 In addition, when individual patient risk calculated from the SII alone was compared with risk calculated by the NHSN multivariable regression models for fusion, revision fusion and laminectomy, the SII score performed comparably at predicting surgical site infection, even as a single variable.15 This suggests that much of the infectious risk conferred to patients undergoing spine surgery is derived from the extent of procedure being performed. The strong predictive ability of SII is likely a result of its collinearity with multiple other significant factors (Table 3-3).
| Variable | Collinearity Coefficient |
|---|---|
| Procedure Type (Fusion, Laminectomy, Revision Fusion) | 0.361 |
| Surgery Duration | 0.674 |
| Posterior Approach | 0.622 |
| Staged Approach | 0.409 |
| Thoracic Level Involvement | 0.439 |
Limitations of Invasiveness Index
Interventions that do not involve decompression, arthrodesis, or instrumentation (e.g., incision and drainage) have not been studied cannot be scored by invasiveness index. In addition, if assessing a relatively homogenous group of procedures (e.g., outpatient microdiscectomy), then the cohort is in effect stratified by “procedural risk” already and the SII scores will be relatively consistent across the cohort. In this case, no association between SII and patient outcomes would be expected.
FUTURE USE OF INVASIVENESS INDEX
As better models are created to predict risk of adverse outcomes in spine surgery, so too will the ability to identify new opportunities for improvement. Risk-adjusted patient outcome measurements allow us to identify which practices are most beneficial, and which may be harmful in the end. As practice standardizes based on available evidence, new opportunities emerge as potential targets for improvement of patient outcomes.
Increasingly, risk-adjusted patient outcome measurements will be performed by third party groups. In this setting, the same epidemiologists, public health officials, or actuaries may be calculating outcome measurements across many different areas in medicine. Promulgation of subject-specific risk scores that underscore the key procedural differences associated with spine surgery (compared to, say, inpatient falls or coronary artery bypass grafting) will be important to ensure that the measurements are truly accurate.
REFERENCES
- Lee NJ, Kothari P, Phan K, et al. The incidence and risk factors for 30-day unplanned readmissions after elective posterior lumbar fusion. Spine (Phila Pa 1976). 2018;43(1):41-48.
- McGuire KJ, Harrast J, Herkowitz H, Weinstein JN. Geographic variation in the surgical treatment of degenerative cervical disc disease: American Board of Orthopedic Surgery Quality Improvement Initiative; part II candidates. Spine (Phila Pa 1976). 2012;37(1):57-66.
- Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol. 2011;32(10):970-986.
- Khaleel MA, Bono CM, White AP, et al. Defining avoidable and unavoidable complications in spine surgery: a survey-based study of spine fellowship directors. Spine (Phila Pa 1976). 2016;41(11):958-962.
- McGregor JC, Harris AD. The need for advancements in the field of risk adjustment for healthcare-associated infections. Infect Control Hosp Epidemiol. 2014;35(1):8-9.
- Mirza SK, Deyo RA, Heagerty PJ, Turner JA, Lee LA, Goodkin R. Towards standardized measurement of adverse events in spine surgery: conceptual model and pilot evaluation. BMC musculoskeletal disorders. 2006;7:53.
- Mehta S, Hadley S, Hutzler L, Slover J, Phillips M, Bosco JA, 3rd. Impact of preoperative MRSA screening and decolonization on hospital-acquired MRSA burden. Clin Orthop Relat Res. 2013;471(7):2367-2371.
- Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1820-1826.
- Maddox JJ, Pruitt DR, Agel J, Bransford RJ. Unstaged versus staged posterior-only thoracolumbar fusions in deformity: a retrospective comparison of perioperative complications. Spine J. 2014;14(7):1159-1165.
- Lee MJ, Cizik AM, Hamilton D, Chapman JR. Predicting surgical site infection after spine surgery: a validated model using a prospective surgical registry. Spine J. 2014;14(9):2112-2117.
- Cizik AM, Lee MJ, Martin BI, et al. Using the spine surgical invasiveness index to identify risk of surgical site infection: a multivariate analysis. J Bone Joint Surg Am. 2012;94(4):335-342.
- Abdul-Jabbar A, Berven SH, Hu SS, et al. Surgical site infections in spine surgery: identification of microbiologic and surgical characteristics in 239 cases. Spine (Phila Pa 1976). 2013;38(22):E1425-1431.
- Friedman ND, Sexton DJ, Connelly SM, Kaye KS. Risk factors for surgical site infection complicating laminectomy. Infect Control Hosp Epidemiol. 2007;28(9):1060-1065.
- Tenney JH, Vlahov D, Salcman M, Ducker TB. Wide variation in risk of wound infection following clean neurosurgery. Implications for perioperative antibiotic prophylaxis. J Neurosurg. 1985;62(2):243-247.
- Hollenbeck BL, McGuire KJ, White AP, Yassa DS, Wright SB. Invasiveness index as a predictor of surgical site infection after spinal fusion, revision fusion, or laminectomy. Infect Control Hosp Epidemiol. 2017;38(1):11-17.
- Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976). 2009;34(13):1422-1428.
- Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98(2 Suppl):149-155.
- Klein JD, Hey LA, Yu CS, et al. Perioperative nutrition and postoperative complications in patients undergoing spinal surgery. Spine (Phila Pa 1976). 1996;21(22):2676-2682.
- De la Garza-Ramos R, Abt NB, Kerezoudis P, et al. Deep-wound and organ-space infection after surgery for degenerative spine disease: an analysis from 2006 to 2012. Neurol Res. 2016;38(2):117-123.
- Dennis HH, Wei DT, Darren KZ, et al. Is Intraoperative local Vancomycin powder the answer to surgical site infections in spine surgery? Spine (Phila Pa 1976). 2017;42(4):267-274.
- Thakkar V, Ghobrial GM, Maulucci CM, et al. Nasal MRSA colonization: impact on surgical site infection following spine surgery.
- Clin Neurol Neurosurg. 2014;125:94-97.
- Watanabe M, Sakai D, Matsuyama D, Yamamoto Y, Sato M, Mochida J. Risk factors for surgical site infection following spine surgery: efficacy of intraoperative saline irrigation. J Neurosurg Spine. 2010;12(5):540-546.
- Boston KM, Baraniuk S, O'Heron S, Murray KO. Risk factors for spinal surgical site infection, Houston, Texas. Infect Control Hosp Epidemiol. 2009;30(9):884-889.
- Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg. 1997;86(6):975-980.
- Radcliff KE, Rasouli MR, Neusner A, et al. Preoperative delay of more than 1 hour increases the risk of surgical site infection. Spine (Phila Pa 1976). 2013;38(15):1318-1323.
- Mirza SK, Deyo RA, Heagerty PJ, et al. Development of an index to characterize the "invasiveness" of spine surgery: validation by comparison to blood loss and operative time. Spine (Phila Pa 1976). 2008;33(24):2651-2661; discussion 2662.
- Mirza SK, Martin BI, Goodkin R, Hart RA, Anderson PA. Developing a toolkit for comparing safety in spine surgery. Instr Course Lect. 2014;63:271-286.