Harold I. Salmons, Jeffrey Ta, Hamadi Murphy, Arjun Sebastian, Kris Radcliff, Alex Vaccaro
INTRODUCTION
Spinal infections involve pyogenic or granulomatous infections of the vertebral column, intervertebral discs, the dural sac or the epidural space. Infections of the spine can take the form of a primary infection of the spine or a spread of microorganisms originating from elsewhere in the body. Spinal infections can also develop postoperatively and most often develop secondary to direct inoculation of the wound. Regardless of the source of the infection, an infection of the spine should be quickly diagnosed in order to prevent structural instability or neurologic compromise. This chapter describes both primary and post-operative spinal infections. Epidemiology, risk factors, etiology, clinical presentation, diagnostic maneuvers, treatment options and prognoses will be explored.
SECTION I: PRIMARY SPINE INFECTIONS
Primary spinal infections are described as infections of the vertebrae that are not secondary to an operation. Such infections commonly originate from elsewhere in the body and spread to the spine and its musculoskeletal components. Pyogenic infections of the spine most frequently involve the lumbar spine (58%), followed by the thoracic (30%) and cervical (11%) regions. Gram-positive organisms such as Staphylococcus aureus and Streptococcus species are the most commonly isolated organisms in patients with pyogenic vertebral infections.1
Pyogenic Infections of the Spine
Primary vertebral osteomyelitis and discitis
Epidemiology
Spine infections are rare infections that can involve the intervertebral disc space (discitis), the vertebral bones, the spinal canal or adjacent soft tissues. Discitis refers to an infection of the intervertebral disc in the spine. Vertebral osteomyelitis refers to an infection of the vertebral bones in the spine. Infection usually is caused by bacterial organisms, but can also be due to viral or fungal organisms. The most common primary spinal infection is pyogenic vertebral osteomyelitis. Vertebral osteomyelitis mostly occurs in adults, with the majority occurring in patients over 50 years old.2The disease may occur from infancy to old age, but has a predilection for the elderly.2–11The incidence may be higher in younger patients that are intravenous drug abusers.12Vertebral osteomyelitis affects men approximately twice as much as women, but the reason for this is not fully understood.13 Vertebral osteomyelitis occurs at an incidence of approximately 2.2/100,000 per year.5,14 That prevalence of this infection increases with age is possibly due to a number of factors: the increasing age of the population, increasing number of patients on renal replacement therapy, increasing number of patients with immunosuppressive medications and increasing rates of bacteremia due to intravascular devices and other forms of instrumentation.13,15,16
Risk factors
Risk factors for infections of the spine involve conditions that weaken the patient’s immune system, such conditions include diabetes mellitus, use of immunosuppressant medications, cancer, HIV/AIDS, malnutrition, history of an organ transplant and intravenous drug abuse.
Etiology
Any condition that causes a bacteremia (even temporary bacteremia such as tooth brushing or venipuncture) may lead to hematogenous vertebral osteomyelitis. The most common sources are urinary tract infections and the transient bacteremia caused by genitourinary procedures.5,17-21 Vertebral osteomyelitis may accompany diarrhea due to salmonellosis,22 otitis media,3 dental extraction,23 infective endocarditis and hemodialysis.24 Spine surgeries, penetrating wounds, chemonucleolysis or discogphaphy may directly inoculate the spine as well.6,25-33
Pathogenesis
There are multiple ways that bacteria can spread into the vertebral column. Three major routes of spread are: (1) hematogenous spread from a distant infection, (2) direct inoculation from trauma, (3) direct inoculation following invasive spinal diagnostic procedures and from spinal surgery. Any condition that causes a bacteremia may lead to hematogenous vertebral osteomyelitis. Osteomyelitis following hematogenous spread of infection is the major mechanism by which adults and children contract vertebral osteomyelitis. Infection spreads into vertebral bodies by first seeding underneath vertebral end plates, which is followed by disc and nearby vertebrae involvement. The extent of this spreading is larger in pediatric spines due to their blood vessels extending into the intervertebral disc, permitting the direct spread of infection to the discs.34 Many now believe that the sluggish flow, scarcity of valves and convolution of the arterial or venous supply leads to vertebral osteomyelitis in patients with bacteremia. Potential sources of hematogenous or contiguous spread of infection include the genitourinary tract, skin and soft tissue (via injection drug use), respiratory tract, infected IV catheter sites, postoperative wound infection, endocarditis and dental infection. In some cases the primary site of infection cannot be identified.35
Since the vascular supply supplying the vertebrae usually bifurcate to supply two adjacent end plates of adjacent vertebrae, hematogenous vertebral osteomyelitis often causes bone destruction of adjacent vertebral bodies and their shared IV disc.36 The lumbar vertebral bodies are most often involved, followed by thoracic and then cervical vertebral bodies.37 Hematogenous sacral osteomyelitis is very rare. Sacral osteomyelitis most often occurs as a result of sacral pressure ulcers; however, it can also follow pelvic infection, trauma and surgeries.38,39 Contiguous spread from adjacent tissues such as the aorta, esophagus or bowel have also been reported. Extension of infection posteriorly can lead to epidural abscess, subdural abscess or meningitis. Lateral extension of infection can lead to psoas, retroperitoneal, subphrenic, paravertebral, retropharyngeal and mediastinal abscesses as well. Epidural or paravertebral abscess development is most common in cases where the invading bacterium is gram-positive rather than gram-negative.40 The spinous process, pedicles, odontoid process and facet joints can also be affected.41 Some patients present with septic facet joints and paraspinous muscle abscesses in the multifidus and longissimus muscles.
Clinical features
Back or neck pain is a common symptom of vertebral osteomyelitis. This pain may radiate to the abdomen, scrotum, leg, groin or perineum. The pain is often worse at night. Therefore, the pain does not resemble the behavior of the typical “mechanical” back pain which is worse with upright position and activity during the day. This atypical pain is usually localized to the affected anatomical area (e.g. cervical, thoracic, lumbar) unless there is multifocal infection. The pain is amplified by percussion to the affected area or physical activity. Pain may be absent in patients with Charcot spine or paraplegia. Neurologic changes such as radicular numbness and muscle weakness may be present in up to one third of patients.
Epidural abscesses may form as a result of posterior spread into the epidural space, and these can cause neurological abnormalities depending on several factors, including the severity of the abscess, the extent of stenosis, the anatomical region and the duration of time present. Epidural abscesses can therefore present with radiculopathy, motor weakness, sensory changes, paralysis or cauda equina syndrome.42 Epidural abscesses can also be asymptomatic.
Patients with lumbar psoas abscesses can present with lumbar plexopathy due to the neural compression. These patients may have weakness with hip flexion, knee extension, sensory abnormalities over the upper thigh and anterior knee, or other neurological abnormalities in the affected nerve distributions. Psoas abscesses can also be asymptomatic.
Diagnosis
Concern for vertebral osteomyelitis is warranted when patients present with new or worsening back or neck pain, particularly in combination with fever, and/ or presence of blood stream infection and/or endocarditis.22,13 Further, clinicians should be suspicious of febrile patients presenting with new neurological symptoms (pain may or may not be present) and patients with new back or neck pain following a recent episode of S. aureus bacteremia.22 Back pain in patients with this diagnosis may respond to bed rest at first, leading to the incorrect assumption that non-infectious causes are present. Additionally, a history of degenerative spine disease may delay diagnosis.13,22
Detecting likely organisms such as S. aureus via CT-guided biopsy of the area in question permits a conclusive diagnosis of vertebral osteomyelitis.43 The diagnosis may be inferred without biopsy in cases of clinical and radiographic findings typical of vertebral osteomyelitis with likely pathogens. Disc space biopsy may be used in certain circumstances where a positive blood culture is detected. Elevated inflammatory markers and suggestive radiographic findings could also suggest the diagnosis when appropriate culture data is not present.
Clinical approach
As a part of a complete history and physical evaluation, patients should be examined thoroughly for neurologic symptoms and questioned about risk factors. Initial tests should include white blood cell count (WBC), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) and blood cultures followed by magnetic resonance imaging (MRI) of the spine.44 Neurological deficits and radiographic evidence of abscess with possible cord compression warrant surgical evaluation.
In the absence of conditions warranting urgent surgical management, patients with radiographic evidence of osteomyelitis should undergo a CT-guided needle biopsy of the affected bone and aspiration of the abscess if one is present.44 Antibiotics should be withheld if possible to avoid contaminating the biopsy. However, in one retrospective study of 92 subjects with vertebral osteomyelitis, giving antibiotics prior to biopsy did not reduce the results of culture yields.45 Cultures for bacteria, fungi and mycobacteria should be obtained as well. In the case of sepsis, empirical antibiotic therapy is acceptable, but otherwise antimicrobial therapy should be withheld until a microbiologic diagnosis is confirmed.22 It should be noted that a needle biopsy may not be necessary in patients with clinical and radiographic findings typical of vertebral osteomyelitis and positive blood cultures with a common pathogen. However, blood culture isolates do not always correlate with culture results from needle biopsy; therefore, needle biopsy should be considered in cases when an alternate source for the bacteremia is evident or suspected.46 If repeat biopsy and initial blood cultures are negative, empirical therapy may follow. If this therapy does not result in objective clinical improvement in three to four weeks, another needle biopsy or preferably an open surgical biopsy should be performed. To rule out noncontiguous lesions, patients should undergo screening imaging of the entire spinal axis.
Treatment
Antimicrobial therapy without surgical intervention is warranted in patients presenting without progressive neurological symptoms. Those that do present with neurologic symptoms should be evaluated for surgery. If cultures are negative but vertebral osteomyelitis is highly suspected based on radiographic findings, empirical therapy initiation is warranted.22 Medications that are appropriate for treating vertebral osteomyelitis vary, and choice of antibiotic should be based on biopsy and culture results. To date, there have been no controlled studies that have compared antibiotic regimens in cases with vertebral osteomyelitis.
If culture/biopsy indicates a Staphylococcal species, in particular methicillin-susceptible Staphylococcus, it is recommended to treat with an anti-staphylococcal penicillin such as nafcillin or oxacillin or cefazolin.13 If the Staphylococcus is methicillin resistant or if the patient is allergic to beta-lactam antibiotics, beta-lactam desensitization or treatment with vancomycin is recommended. Ceftriaxone should not be used as an alternative to cefazolin or anti-staphylococcal penicillin in the absence of special circumstances, such as inability to give multiple daily IV doses.
If the bacterium is found to be of the Streptococcal species and if the streptococci is sensitive to penicillin, it is recommended to treat with either ceftriaxone or penicillin G. If the Streptococcus has intermediate susceptibility to ceftriaxone, then higher doses of penicillin may be used. Specialized in vitro testing through an infectious disease specialist may be necessary for patients with fully resistant streptococci. Empirical therapy targeting the common pathogens using is warranted for gram-negative bacteria.
To treat infection due to Propionibacterium acnes it is recommended to treat with penicillin or vancomycin.
Fungal vertebral osteomyelitis, particularly those of the Candida fungal organism are difficult to treat because the diagnosis is often delayed for weeks to months.47,48 The infected bone is often poorly perfused, which limits both the delivery of antifungal drugs and the host’s own inflammatory response.49 Furthermore, foreign material may be present as a result of procedures like median sternotomy.47,48 Few data are available on the penetration of antifungal agents into bone.50 Despite the treatment challenges for vertebral osteomyelitis due to fungal infections, treatment suggestions for osteomyelitis caused by fluconazole-susceptible Candida species such as C. albicans include oral fluconazole daily for 6 to 12 months or an IV echinocandin daily for at least two weeks followed by oral fluconazole for a total of 6 to 12 months.25
Alternatively, a lipid formulation of amphotericin B, 3 to 5 mg/kg IV daily, can be administered for at least two weeks followed by oral fluconazole for 6 to 12 months. This regimen should be followed carefully due to the nephrotoxicity of amphoceterin B formulations. These treatment recommendations are all based off of recommendations from reports and case series.25
The treatment for fluconazole-resistant infections caused by organisms such as C. glabrata is more limited than those caused by C. albicans.47,51–53 Due to its resistance to fluconazole, the preferred treatment of C. albicans osteomyelitis are echinocandins followed by step-down therapy with oral voriconazole as long as the isolate is susceptible.3,54,55 Due to the chance of cross-resistance between voriconazole and fluconazole, susceptibilities must be understood beforehand. The best experiences in treating hematogenously spreading C. glabrata infections is with amphotericin B.51-53
In a review of 207 cases of Candida osteomyelitis reported between 1970 and 201, 44 percent of subjects were treated with antifungal therapy alone, 5 percent with surgery alone and 48 percent with both surgery and antifungal therapy.56 Among the 92 patients treated with antifungal agents alone, 97 percent had a positive response, but 16 (17 percent) relapsed. There was no reported benefit of one antifungal treatment over another in this study. Notably there was a relapse rate of 43 percent among patients receiving both antifungal treatment and surgery. The authors postulated that many patients relapsed as a result of a therapy that was too short in duration. It is difficult to determine why the relapse rates were so high without further information about the type of antifungal used and the duration of treatment.
Empirical therapy
In certain contexts, empirical treatment of patients with vertebral osteomyelitis is warranted. Gram-negative stain and culture results in particular necessitate treatment with an empirical regimen of antimicrobials that are effective against the most common sources of vertebral osteomyelitis, including staphylococci, streptococci and gram-negative bacilli. Such treatment consists of vancomycin and cefotaxime, ceftazidime, ceftriaxone, cefepime or ciprofloxacin. Though rare, if patients present with positive anaerobic cultures, such coverage is warranted and would involve metronidazole added to the above treatments.57 Duration of therapy varies from patient to patient, but in general it is recommended to treat for 6 weeks but may be extended for more resistant organisms.57–59 Follow-up should be based on the response to therapy and the overall health of the patient.
Surgical intervention
Surgical debridement plays an important role in the treatment of many of these infections. The only absolute indication for surgery is a progressive neurological deficit or sepsis. Relative indications for surgery include:
- Failure of medical management (including six weeks of IV antibiotics with which the patient has been compliant)
- Progressive bony destruction
- Static neurological deficit that has not improved
- Fracture
- Instability
- Recalcitrant pain
- Spinal cord level (cervical or thoracic) compressive pathology
Prognosis
The most serious complication of vertebral osteomyelitis is neurologic impairment secondary to abscess formation or bony collapse. Back pain may fade with therapy, or it may persist. Minimizing the time between the onset of symptoms and the initiation of appropriate therapy is the best way to reduce morbidity and mortality. Mortalities due to vertebral osteomyelitis have decreased to less than 5 percent, and the rate of residual neurologic deficits among survivors is less than 7 percent.22
In a retrospective study including of 260 patients with vertebral osteomyelitis that had long-term follow-up, 11% of patients died, while residual complications and disability occurred in 33%. Relapse occurred in 14% and S. aureus was associated with increased risk for relapse diagnosis.60 S. aureus infection was associated with increased risk of relapse. Complications included neurological deficit (16%) and persistent back pain (32%).
A retrospective study of 253 patients with vertebral osteomyelitis investigated the long-term outcomes in such cases with a median duration of follow-up 6.5 years.60 Relapse occurred in 14% of patients, and residual symptoms occurred in 31%; 11% died. Surgery was performed in 43% of patients and was successful in 79% of cases. Results of this study should be interpreted with caution, since cases were collected over a long time period, (median duration of follow-up was 6.5 years) which means that some cases occurred prior to the development of modern diagnostic modalities. A longer time to diagnosis, neurologic compromise and hospital acquisition were identified by the authors as major independent risk factors for the adverse outcomes of vertebral osteomyelitis.
Conclusion
Vertebral osteomyelitis occurs via hematogeneous spreading from a distant site of infection, direct inoculation from trauma or spinal surgery, and contiguous spread from adjacent soft tissue infections. Staphylococcus aureusis the most responsible organism for vertebral osteomyelitis. The major clinical manifestations of vertebral osteomyelitis are spinal pain, with or without fever. Pain to percussion over the infected area is a common physical finding. Elevated erythrocyte sedimentation rate and C-reactive protein are the two most common laboratory abnormalities. MRI is the most sensitive imaging modality with which vertebral osteomyelitis can be detected. Vertebral osteomyelitis can be confirmed by biopsy of the infected intervertebral disc space or vertebral bone. Blood cultures are positive in a majority of patients, and a biopsy may not be necessary for patients with clinical and radiographic findings typical of the infection along with positive blood cultures with a likely pathogen. Most cases of vertebral osteomyelitis respond to antimicrobial therapy, and surgery is necessary in a minority of patients with vertebral osteomyelitis. Prompt surgery is warranted for patients with a progressive neurologic deficit or septicemia. Surgery may be considered for other indications including epidural or paravertebral abscesses and/or cord compression. Antimicrobial therapy should be withheld until a microbiologic diagnosis is confirmed. The exceptions to this rule are neurologic compromise and sepsis where empirical antibiotic therapy is useful. Duration of antibiotic treatment for bacterial infection is typically 6 weeks at a minimum, and longer durations are warranted in a case-specific manner.
Epidural abscesses
Epidemiology
Epidural abscess is an uncommon but serious pyogenic infection with significant morbidity and mortality. Abscesses that are entrapped within the spinal column can impinge and compress the neurologic structures and cause immense issues and even death. Spinal epidural abscesses (SEA) are more common than intracranial epidural abscesses.61 Although SEA is an uncommon disease, evidence suggests that incidence may have increased over the last few decades.62,63 From 1975 to 1998, for example, the incidence increased from 0.2-1.2 to 2.5-3.0 cases per 10,000 hospital admissions.64 This increase may be due to an aging population or increased sensitivity of modern diagnostic modalities, particularly the increased sensitivity of magnetic resonance imaging (MRI).65
A study reported the first published population-based incidence of spontaneous epidural abscess. During a ten year period, the occurrence in a county in Minnesota was 0.88 case/100,000 person-years (95% CI 0.27-1.48).66 Additionally, another report found that radiographic evidence of an epidural abscess was reported in 64 of 167 patients (38%) with hematogenous vertebral osteomyelitis due to methicillin-susceptible S. aureus (MSSA) or gram-negative bacilli.40 Epidural abscess was more common in patients with MSSA than it was in patients with gram-negative bacilli (47% versus 29%). SEAs are most prevalent between ages 50 and 70.64
Risk factors
Multiple medical comorbidities have been shown to be risk factors for SEAs, including diabetes mellitus, tattoos, intravenous drug abuse, alcoholism, HIV infection, trauma, acupuncture, vertebral osteodiscitis, bacteremia, hemodialysis and paraspinal injections.67,68 There is also a risk of SEA with epidural catheter placement. Epidural catheter placement has demonstrated an incidence of abscesses ranging from 0.5 to 3 percent.4,69–71 A review of over 500,000 pregnant women undergoing epidural catheter placement in the United Kingdom had only a single SEA event occur, which suggests that SEA risk is potentially lowered when catheters are placed for shorter durations of time.72 In considering a diagnosis of SEA, these risk factors may often be absent, and this poses a challenge to reaching an early detection of SEAs.
Etiology
The main bacteria that causes SEAs is S. aureus, which accounts for about two-thirds of cases caused by pyogenic bacteria.42,62,73–75 Though the main cause of SEAs is S. aureus other causative organisms include gram-negative bacteria, streptococci and coagulase-negative staphylococci.42,62,73 In various parts of the developing world, Mycobacterium tuberculosis is a more frequent cause of SEAs.76 It will be discussed in the section on Granulomatous Infections of the Spine.
Skin and soft tissue infections as well as complications of spinal surgery, epidural catheters left in place for pain control and invasive procedures account for the most common origins of infection. SEA infections due to epidural catheters can be a result of contamination at the time the catheter is inserted, by bacteria ascending through the catheter from normal skin flora following insertion, from injection using contaminated syringes or local anesthetic solutions, or by hematogenous seeding from bacteremia while the catheter is in place.77
Infection control is crucial when placing catheters and performing procedures that may place patients at risk for SEAs. The importance of infection control measures to prevent SEAs associated with catheter placement was illustrated by a report indicating the importance of facemasks and sterile technique while epidural catheters are being placed.
In this study, the infecting organism was isolated from nasal swabs taken from the physician who placed the catheter.78
Pathogenesis
Bacteria can gain access to the epidural space via the blood or by direct inoculation into the spinal canal or by direct extension such as from vertebral osteomyelitis or a psoas abscess or a septic facet joint. Focal pyogenic lesions often precede SEAs, and these involve the vertebral disc or the junction between the disc and the vertebral body (pyogenic infectious discitis).26,79 As the pyogenic inflammation progresses and the abscess extends into the epidural space, damage can be caused to the spinal cord. Although the full length of the spinal column may be affected, longitudinal extension is most common between three to five spinal segments when infections are in epidural spaces.74 SEAs sometimes do occur at noncontiguous lesions.
Despite the higher chance that epidural abscesses occur at contiguous sites of the vertebrae, they can sometimes occur at noncontiguous locations in the spine. Skip lesions were shown to occur in a retrospective study in 9% of adult patients with SEAs.80 Such noncontiguous lesions were more common in patients with a delay in presentation (a week or longer), a simultaneous area of infection outside of the spine or paraspinal region, and an ESR of greater or equal to 95 mm/hour at presentation. The authors suggested the use of these three parameters as indications for skip lesion presence, but more data must be obtained to verify this finding. At the time of surgical drainage, it is common to find granulation tissue within the abscess if the abscess has been present for more than two weeks. In acute cases, epidural abscesses may contain frank pus.
Clinical presentation
The clinical presentation of SEAs is variable but classically involves fever, spinal pain and neurologic insufficiencies. However, only a small portion of patients present with all three of these symptoms at presentation.27,81,82 Commonly patients will initially present with severe back pain alone. This may eventually be followed by radicular symptoms, weakness, bowel/bladder dysfunction, or even paralysis.74
Spinal Epidural Abscess is very difficult to diagnose using routine lab work due to a lack of specificity in markers as well as a diverse array of lab values in patients with SEAs. The hematocrit is often normal and the leukocyte may be high or normal. Additionally, the ESR is often high in both SEA and vertebral osteomyelitis. In an emergency department patient population, only 60% of patients that were diagnosed with SEA had leukocytosis at their initial visit.27 In contrast, other studies have shown the ESR to be elevated (>20 mm/hour) in 100% of patients with SEA.27,83 C-reactive protein (CRP) has also been shown to be elevated in as high as 87% of patients with SEA. However, while the sensitivity of CRP and ESR is high, it is important to keep in mind that as many as 33% to 50% of patients with spinal pain not related to SEA can have elevated markers suggesting less specificity for these diagnostic tests.
Diagnostic approach and imaging
SEA is a rare cause of back pain, and thus is a diagnosis of exclusion. Once the diagnosis of SEA is considered, it is important to image the spinal column using MRI. MRI is the preferred test due to its ability to detect infection early in its course as well as provide higher level of visualization of the extent of the infection75,84 (Fig. 1-1 to 1-4). Isolating the causative organism is a proper subsequent step to detection, and CT-guided needle biopsy directly obtained from the epidural abscess is beneficial.85
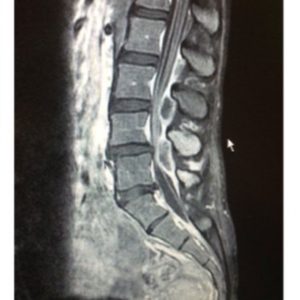
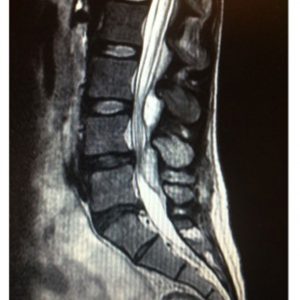
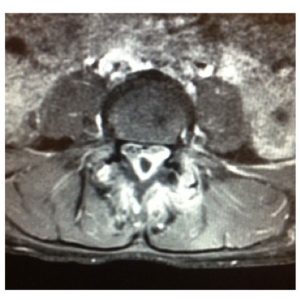
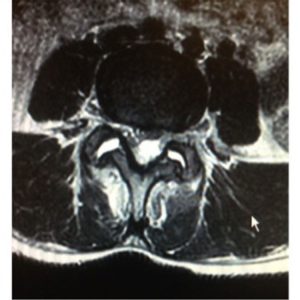
Determining epidural soft tissue edema versus epidural abscess is critical. MRI can make this distinction. Classic characteristics of SEA using MRI as the preferred imaging modality have been well described and are quite distinctive.40,86–91 Abscesses tend to have low or intermediate intensities on T1-weighted MR sequences and high or intermediate intensity on T2-weighted images. Hyperintensity is usually visualized within the fluid portion of the abscess on T2-weighted images and hypointensity on T1-weighted images.92 A series of case reports has shown that Gadolinium-enhanced MRI imaging is useful in determining the age and consistency of an SEA (pus or granulation tissue)92. Liquid pus reveals itself as an area of low signal intensity on T1-weighted images, whereas a rim of tissue that enhances after the injection of Gd is representative of granulated tissue.40,86,92 Comparatively, epidural soft tissue edema usually manifests as diffuse hypointensity of T1-weighted images, hyperintensity on T2-weighted images, and contrast enhancement. Some studies have suggested that the presence of ring-like or linear dural enhancement is an indication for surgery.28,93 The authors believe that CT with contrast is not nearly as sensitive as MRI in making the diagnosis or determining the extent of infection or the degree of stenosis. Myelography is contraindicated in the setting of an active epidural infection.
Differential diagnosis
S. aureus is not unique to SEAs. It is the major pathogen in vertebral osteomyelitis and discitis as well,67 so the combination of back pain and infection by the major pathogen are not specific to SEA. SEAs share symptoms with a variety of conditions, and this warrants the consideration of the following diseases as differential diagnoses: metastatic tumors, disc and degenerative bone disease, vertebral discitis and osteomyelitis, meningitis and herpes zoster before to the appearance of skin lesions.61
Treatment
Treatment for spinal epidural abscesses involves reducing the size, eliminating the mass, and eliminating the pathogenic organism. A combination of aspiration, drainage and antibiotic regimens are useful in achieving these goals. Medical management may be considered in neurologically stable patients with an epidural abscess in the lumbar spine that are in a monitored setting and are receiving IV antibiotics. If extremity weakness is present, surgical intervention should be considered in the absence of any contraindications.75,94 In the setting of significant weakness or paralysis surgery should be performed as early as possible.67 The earlier one performs surgical decompressions and drainage the better. If the MRI findings indicate spinal cord compression or severe stenosis, even in the absence of signs or symptoms or neurologic compromise, surgery
should be considered. Patients with a neurological deficit should be placed into an ICU setting with a maintained mean arterial pressure >80, and carefully monitored. Intravenous steroids should be avoided.
Though targeted therapy to a causative organism is preferred, empirical therapies targeting likely pathogens are more often employed due to a lack of controlled evidence around the best antimicrobial treatments of SEAs. Appropriate empirical IV regimens include vancomycin for empirical MRSA coverage plus either cefotaxime or ceftriaxone or cefepime or ceftazidime. Pseudomonas aeruginosa is associated with intravenous drug abuse,64 and when this pathogen is suspected one should use cefepime or ceftazidime. Subsequently, once a known pathogen is identified via culture, the treatment regimen should be simplified to antibiotics directed against those specific pathogens.
If the organism is determined to be S. Aureus, vancomycin should be used.95,96 However, a drawback to vancomycin is that it poorly penetrates cerebrospinal fluid (CSF).96,97 If MSSA is discovered, treatment should be changed to nafcillin or oxacillin. Vancomycin should be continued if the organism is methicillin resistant. In the case of poor vancomycin CSF penetration, drug penetration and efficacy can be augmented by adding rifampin to vancomycin because it achieves bactericidal concentrations in the CSF.95,96,98
Duration of therapy for a patient with SEA should occur on a patient-by-patient basis. The duration of antimicrobial therapy generally provides cure in 4-6 weeks of treatment.61,99 Follow up MRIs should be performed as needed. For patients who also have osteomyelitis associated with an SEA, therapy should continue for approximately six to eight weeks.62
The primary forms of surgical intervention are decompression of neural tissue and stabilization of bony elements of the spine. Another option is decompression alone.74,88,100,101 The main factor in deciding the surgical approach is the location of the abscess. At the time of surgery, a biopsy should be taken to identify a causative organism. As with the trend of infections of the spine, surgery should be performed as quickly as possible in the setting of a neurologic deficit. Those treated non-surgically continue to exhibit high failure rates (41-42.5%) accompanied by increased morbidity (up to 22% permanent paralysis) and mortality (rates from 1.8% to 25%).102–108 Comorbidities worsen the overall prognosis in patients with SEAs. They predispose these patients to a higher incidence of perioperative complications after surgery, particularly if they have a neurological deficit.
Prognosis
Outcomes are often measured by examining mortality and recovery rates from neurological deficits.64 The prognosis for patients with SEAs is generally poor. Comorbidities enhance the risk of death, and patients generally die from sepsis or other complications. About 5% of patients with SEA die due to sepsis or other complications.64,67 In a retrospective study, permanent paraplegia has been observed to occur in 4%-22% of patients. Neurologic recovery is unlikely if paralysis is present for greater than 24 hours before surgery.19,42,67,74,103,109 However, it is unclear without prospective, randomized data (which would be impossible to perform due to ethical concerns) whether earlier surgery would have reduced the incidence of permanent paraplegia or whether patients who go to surgery late are simply more likely to have a poor prognosis due to other confounding medical factors. In some patients, the level of neurologic recovery post-op is related to the duration of the neurological deficit. However, the neurological recovery is also related to the severity of the initial deficit and there are many patients who have early surgery who still have a persistent postoperative deficit. In a population of emergency department patients previously mentioned, 37% of patients with an SEA had residual motor weakness after surgical debridement27. Delayed diagnosis was associated with increased risk of residual weakness compared to patients with no delay in diagnosis (45% versus 13%). However, even in patients who had an early diagnosis, there was still a high rate of permanent neurological deficit.
Conclusion
Epidural abscess is a rare infection that can cause a neurologic deficit due to compression of the spinal cord. Quick diagnosis and proper treatment are critical to avoid complications and to improve the chances for eradication of the infection. Bacteria can enter the epidural space in a variety of ways. They can spread hematogenously, by direct extension from infected contiguous tissue, or via direct inoculation into the spinal canal by various procedures. Factors that increase the risk of developing SEAs are epidural catheters, diabetes mellitus, alcoholism, HIV infection, bacteremia and IV drug use. The most common causative pathogen of SEAs is staphylococcus aureus, as it is responsible for two-thirds of cases caused by pyogenic bacteria. Approximately one-third of patients have no identifiable source of their infection. In the two-thirds of patients where a path of entry was identifiable, the most common sites of origin are infections of skin and soft tissues and complications of spinal surgery or other invasive procedures, including epidural catheters that are left in place for pain control. SEA can initially manifest itself through a fever and general malaise. The classic diagnostic triad of SEA is fever, back pain and neurologic deficits although presentations vary significantly. Obtaining an MRI early is critical to evaluating the extent of the infection and planning treatment. Prompt surgical decompression of epidural abscesses that meet surgical criteria is critical to avoid a worsening neurologic deficit, maximize outcomes and successfully treat the infection.
Granulomatosis infections
Granulomatosis infections may be caused by fungi, bacteria and spirochetes. Due to their uncommon nature and similar clinical and histological features to each other, they have been grouped together.
The most common granulomatous infection of the spine is tuberculosis. Tuberculosis spondylitis (Pott’s Disease) will be described in detail while fungal and other granulomatous infections will be reviewed by outlining differences between them and tuberculous infections. Bacteria in the order of Actinomycetales cause chronic infections. This order includes Mycobacteriaceae, Actinomycetaceae and Nocardiaceae.
Tuberculosis
Epidemiology
Tuberculosis (TB) is a disease that dates to ancient times. Signs of skeletal TB have been reported from as early as 8000 BC in Europe from Neolithic remains.110 Skeletal tuberculosis accounts for approximately 10-35% of cases of extrapulmonary TB.111-115 The most common form of skeletal TB is tuberculosis spondylitis (Potts Disease), which comprises about half of musculoskeletal TB cases.116 The incidence of tuberculous spondylitis varies considerably throughout the world and is usually proportional to the quality of public health services available. This illness is more common in underdeveloped countries where malnutrition and overcrowding are major problems. In affluent countries, the incidence has decreased in the last 30 years, and it is now uncommon.117 A neurologic deficit will develop in 10-47% of patients with Potts disease.118-129 In developing countries, the disease is still a significant source of morbidity and mortality. In Asia and Africa, a large percentage of patients are children.1,21,128–134
Etiology
Spinal tuberculosis may occur from hematogenous dissemination from a primary focus. The pulmonary and genitourinary systems are the most frequent routes of infection, but the disease may also arise from other skeletal lesions.125,135,136 The infection reaches the skeletal system through vascular channels, generally the arteries, as a result of bacteremia, or in the axial skeleton through Batson’s plexus of veins.137,138
Pathogenesis
The route of spread of early spinal TB is similar to that of other pyogenic infections of the spine. During primary M. tuberculosis infection, bacteremia may lead to seeding of organisms in bone and/or synovial tissue which may subsequently spread upon failure of host immune defenses.139 After the primary infection, a foci of infection may be contained by the cellular immune response. Mycobacterium tuberculosisis the most common pathogen.
Active tuberculosis (TB) disease can develop quickly or very slowly. In highly pervasive areas, musculoskeletal TB often presents in young patients during the year following primary lung infection. Later reactivation of infection is more common outside endemic areas, and this occurs mostly in adults. The three main types of spinal involvement are peridiscal, central, and anterior.140 Posterior tuberculosis involvement is uncommon.
Clinical presentation
In the classic presentation of tuberculosis spondylitis, the patient complains of local back pain and tenderness, gibbus and a cold absess and a prominent spinal deformity.140 In industrialized countries, presentation is generally early but in underdeveloped countries the disease can be far more advanced and involve more severe symptoms such as paraplegia, kyphosis and draining sinuses.1,126,136,141 Neurologic complications are common in the thoracic and cervical regions and are present at a rate of 23 to 76%.142
The lower thoracic and upper lumbar regions are most commonly affected by Pott’s disease.132,140,143 Adjacent vertebrae may be affected. Once two adjacent vertebrae are involved, the infection spans the adjoining intervertebral disc space. Compared to bacterial vertebral osteomyelitis, this process usually occurs later in Pott’s disease and may have the appearance of relative disc sparing. However, the bony endplates and vascular channels are affected, and thus the disc tissue eventually undergoes necrosis. In some infections, this may lead to intervertebral disk space narrowing and ultimate collapse of that space. This collapse may result in spinal kyphosis and ultimate neurologic compromise.144 Soft tissue masses are common at the site of infection, and delayed diagnosis of Pott’s disease is a problem in endemic areas.138,145
Diagnostic evaluation and imaging
There are a number of radiographic findings that can help a physician detect TB spondylitis in their patient. Radiographic abnormalities are usually first observed in the anterior aspect of a vertebral body and are characterized by demineralization of the end plate and a loss of definition of the bony margins.146 Similar to pyogenic infections, peridiscal involvement and subsequent bone destruction may be evident. The initial radiographs often show far advanced bony changes, in contrast to pyogenic infections in which radiographs may be normal on first presentation. Central body involvement can look like a tumor and cause greater and earlier bone collapse than peridiscal involvement.140 Occasionally, lumbar spine radiographs will demonstrate calcification in the psoas muscle in cases with a long standing abscess.120 Additional presentations include the presence of a paravertebral abscess and pulmonary involvement as observed by a chest radiograph. In some patients, spinal TB presents with osteolytic lesions occurring at multiple sites in the absence of disc space involvement. Spinal TB without disc involvement can be observed in approximately 50% of cases.147
Spinal tuberculosis is a challenging disease to diagnose, as it resembles other disease entities. The greatest challenge is considering the diagnosis since there is no evidence of active disease in more than half of cases. A person’s country of origin obtained through the history may help with the diagnosis. The ESR generally is increased but is nonspecific. A tuberculin purified protein derivative test (PPD) is usually positive and indicates present or past exposure to Mycobacterium.132 Though such tests may be useful in reaching a confirmatory diagnosis, a recent, retrospective study of 145 treated spinal TB cases reported that 85% of cases with tuberculosis of the spine can be diagnosed through MRI alone.
Tuberculosis of the spine is difficult to distinguish from pyogenic infections of the spine as rim enhancement has been reported to suggest both tuberculosis and pyogenic spondylitis.148 Two reported useful findings on MRI that are characteristic of Pott’s is thin and smooth enhancement of the abscess wall and well-defined paraspinal abnormal signals.148 These findings are in contrast with the thick and irregular enhancement of abscess wall and paraspinal abnormal signal that are suggestive of pyogenic spondylitis. Additionally, tuberculous spondylitis has been reported to show a subligamentous spread to three or four vertebral levels and multiple vertebral or entire body involvement.
Therefore, contrast MRI is essential for distinguishing between these two common infectious sources.
Differential diagnosis
The differential diagnosis of Pott’s disease includes degenerative disc and facet joint disease, vertebral body collapse due to osteopenia, spondyloarthropathy, pyogenic spinal infection and malignancy. These each can present with similar clinical findings. Most of these conditions can be distinguished with imaging studies.139
Treatment
Antituberculous treatment for patients with spinal TB should be started as early as possible. Eradicating the infection and preventing a neurological deficit and spinal deformity are the primary goals of management for patients with spinal TB. The drug regimen depends on whether or not the patient has drug-resistant TB or is HIV positive.
The optimal duration of therapy for treatment of spinal TB is not known. For most patients receiving first-line agents (isoniazid, rifampin, pyrazinamide and ethambutol), six to nine months of therapy is satisfactory.149 A longer duration of therapy (9-12 months) is necessary for patients whose regimens do not include rifampin and/or who have extensive disease.149,150
Surgery
Surgical intervention is necessary for patients with spinal disease and severe static neurological deficits (e.g., paraplegia) on presentation and destabilization of the spine.149
Decompression, use of hardware for stabilization of spine, abscess drainage, and debridement of infected tissue are common surgical procedures that are performed.132,151 Due to advances in technology, minimally invasive surgical approaches are now possible through video-assisted thoracoscopic anterior surgeries, and these procedures have been used successfully to manage patients with neurological symptoms who may or may not also have prevalent bony destruction involving the thoracic or lumbar spine.152
In general, abscess drainage is universally indicated only if the patient is septic from the abscess or the abscess is extensive and/or results in a neurologic deficit. Paravertebral abscesses in the thoracic spine can be drained effectively by a costotransversectomy.153 Large psoas abscesses may be drained by a retroperitoneal approach.131 Tuberculous abscesses are not generally liquid in nature and are characterized as phlegmonatous.
Spinal TB is predominately an anterior spinal disease. Cord compression by granulation tissue, casseous material and abscess largely occurs in the front of the spine. The anterior surgical approach was popularized by Hodgson et al.154 and Benli et al.155 in a series of 100 patients. They reported 19.8°±7.3° correction of kyphosis with a preoperative local kyphosis angle of 22.5°±11.0° (range, 10°–80°) improving to a postoperative angle of 2.7°±3.9° (range, 0°–22°). The latter study reported no apparent pseudarthroses, and all
patients healed without recurrence and reactivation.155 An anterior approach with grafting and instrumentation can be done as a stand-alone procedure in some cases minimizing blood loss, operative time and graft dislodgement. Posterior instrumented fusion can be done after anterior decompression either as a two-stage or single stage procedure.156,157 In contrast, other authors have described effective treatment of spinal TB using all posterior approaches with transpedicular, lateral extracavitary or costotransversectomy approaches to gain access to the anterior column of the spine.157 These techniques avoid possible complications of violating the thoracic or abdominal cavities.158 However, these approaches require technical expertise to avoid untoward neurologic or vascular complications.159
While the ideal surgical approach depends on surgeon experience and disease extent, a recent comparative study of surgical management by a posterior only and combined posterior and anterior approaches for thoracic spinal TB in the elderly concluded that the posterior only procedure had better clinical outcomes.157,160 Another study comparing anterior and posterior approaches in the treatment of thoracic and thoracolumbar TB found that both approaches are comparable with regards to duration of surgery, blood loss, fusion and functional outcomes. However, the posterior approach was associated with significantly better kyphosis correction.157,161
Continuous destruction of the anterior column and progressive anterior collapse of the spine by TB despite anti-tubercular treatment may result in kyphosis. After healing of the spinal infection, the post-TB kyphosis in adults may be static, but kyphosis progresses dramatically in 40% of pediatric cases.157,162 Since the advent of posterior-only vertebral column resection (PVCR) by Suk et al.,163 great results have been reported from investigators using PVCR for tubercular deformities of the spine,158,163–169 A study of 17 patients reported an average kyphosis correction rate of 68.7° ±6.5°.164 While PVCR is a powerful deformity correction technique, complication rates as high as 40% have been described in the literature.170 This procedure is recommended only for severe kyphosis either in active or healed disease.
New techniques in minimally invasive spine surgery are emerging for the treatment of various degenerative spinal disorders. Video-assisted thorascopic surgery (VATS) was developed to decrease the approach related morbidity associated with thoracotomy. VATS is a great alternative to conventional thoracotomy with minimal morbidity. Previous work has examined the role of VATS in spinal TB. Good clinical, neurological and fusion outcomes have been reported in VATS treated tubercular spondylitis.171 VATS-assisted surgical decompression is a safe and effective means for anterior debridement and fusion in tuberculosis of the spine in select patients.155,172 The efficacy of minimally invasive spinal decompression through an interlaminar approach combined with local chemotherapy in patients with thoracic or lumbar TB and spinal epidural abscess has also been examined.173 A minimally invasive spinal canal decompression, focal debridement, and catheter drainage through a posterior interlaminar approach has been described. Satisfactory patient outcomes with this minimally invasive approach, in combination with local and systemic chemotherapy, have been shown.
Prognosis
With tuberculous spondylitis, the prognosis is generally good, yet it depends on the age and general health of the patient, the severity and duration of the neurologic deficit, and the treatment selected. A study used logistic regression analysis to evaluate outcomes of 43 patients with Potts paraplegia at six months. This group found that the most important prognostic factor that predicted six-month outcome included initial muscle power, paraplegia score, sensory-evoked potentials (SEPs) and motor-evoked potentials (MEPs).174 Patients with lower paraplegia scores and mild weakness were more likely to recover completely by six months than more severe cases. Another study of 129 subjects was conducted in an endemic region that showed recovery in 70% of patients within 6 months of treatment.175
Conclusion
Tuberculosis spondylitis (Pott’s disease) is the most common form of skeletal TB, and it usually affects the lower thoracic and upper lumber region. Once two adjacent vertebrae are involved, infection can involve the adjoining intervertebral disc space, yielding vertebral collapse. Consequential kyphosis can lead to cord compression and paraplegia. The most common symptom of Pott’s is local pain that increases in severity over weeks to months. Muscle spasm and rigidity are also common. Constitutional symptoms such as fever and weight loss are relatively uncommon. Diagnosis of spinal TB is established by culture of infected tissue, which is obtained through open or aspirate biopsy.
Radiographic imaging, particularly MRI, is useful in identifying Potts disease as it allows for imaging of bony and muscular tissue. Treatment of Potts disease consists of antituberculous therapy similar to the regimens for pulmonary tuberculosis. Optimal duration of therapy is uncertain. For patients receiving first-line agents in the absence of extensive disease, 6 months of therapy is suggested. A longer duration of therapy, 9-12 months, is warranted for patients on regimens that do not include rifampin and/or for patients with extensive or advanced disease. Surgical intervention is warranted in the presence of neurological deficits and/or worsening deficits while on appropriate therapy, as well as for patients with spinal disease and deformity at presentation.
Actinomycosis
Actinomycosis israelii is a slowly growing, facultative or anaerobic, filamentous, gram-positive bacteria. The disease expresses itself in a manner similar to that of true fungi. Actinomyces species are found in the flora of the oral cavity and vagina and may cause infection with abscess formation and draining sinuses. The disease is noted for causing chronic suppurative infections with external sinuses that discharge distinctive aggregates of organisms (“sulfur granules”).176,177 Surgery, infection or trauma are required by the organism for penetration into the mucosa.
Hard, fibrotic walls typically characterize lesions, with granulation tissue surrounding regions of pus. Sinus tracts extending to the skin or into other organs are often seen in more chronic infections.176 The most classic presentation of the disease is the cervico-facial form, accounting for approximately half the cases, followed by thoracic, abdomino-pelvic and cerebral invasions.178 In much rarer cases, A. meyeri has been identified in the spine. It is usually secondary to infection of contiguous tissues rather than to hematogenous spread.178–180
The diagnosis of actinomycosis is challenging as it can mimic other diseases such as malignancies, and the procedures required for accurate microbiological identification of the pathogen are challenging. The main differential diagnoses include all chronic and suppurative infectious processes such as nocardiosis, tuberculosis, spondyloarthritis and primary or secondary malignancies.178 Actinomycosis usually spares intervertebral discs and involves adjacent pedicles and transverse processes, as well as neighboring ribs.178,179 Microbiological diagnosis requires culturing clinical specimens such as pus, enriched in culture media and in appropriate culture conditions. Actinomyces species appear as “molar-tooth” colonies on agar.178 Species identification is achieved through complex phenotypic testing and mass spectrometry or 16S rRNA sequencing. Penicillin G is the best treatment of actinomycosis; however, Actinomycosis species are susceptible to various antimicrobials including tetracyclines, erythromycin, clindamycin, cephalosporins, carbapenems, streptomycin and chloramphenicol.178 A long duration of treatment is suitable to prevent relapses, and a combined medico-surgical approach is required for complicated disease forms.178
Brucellosis
Brucellosis is caused by an aerobic, gram-negative coccobacillus commonly found in domestic animals. Brucellosis is transmitted to humans by direct contact, by ingestion of contaminated products and by inhalation of aerosols.181–184 Brucellosis is one of the most widespread zoonoses worldwide.185 Brucellosis is a systemic infection with a broad clinical spectrum, ranging from asymptomatic disease to severe and/or fatal illness.186 The main presentations are acute febrile illness, with or without signs of localization, and chronic infection. Focal infection occurs in about 30 percent of cases, and brucellosis can affect any organ system.186–189 Osteoarticular involvement is the most common presentation. The sacroiliac joints and large joints of the lower limbs are most frequently involved.190–193 Spondylitis is a serious complication of brucellosis, and it is more prevalent in older patients and patients with prolonged illness prior to treatment. Spondylitis occurs more frequently in lumbar vertebrae.12,194,195 Paravertebral, epidural and psoas abscess can occur in the context of brucellar spondylitis.12,195
The characteristic radiologic features of brucellar spondylitis include predilection for the lower lumbar spine, intact vertebral architecture despite evidence of diffuse vertebral osteomyelitis, disc space involvement, minimal associated paraspinal soft tissue involvement, and absence of gibbous deformity.184,196 MRI findings are similar to TB, except that TB often produces more severe changes with more deformity and abscess formation.196
Treatment for Brucella spondylitis should consist of two antibiotic agents for at least 12 weeks. Patients respond better to doxycycline plus streptomycin than to doxycycline-rifampin.194,197 The duration of therapy is at least as important as the choice of
antimicrobial agents. In one meta-analysis, the failure rate for patients treated less than 6 weeks was 43%; the failure rate for patients treated more than 12 weeks was 17%.198 Surgery may be warranted in the context of spinal instability, persistence or progression of neurological deficit, vertebral collapse or localized abscess (epidural or paravertebral).186,199
Fungal infections
The fungi that cause disease in humans can be classified as either opportunistic or pathogenic fungi. The opportunistic fungi (Aspergillus, Candida, Mucorspecies) rarely cause disease in healthy people, but do cause infection in immunocompromised hosts. Pathogenic fungi (Blastomyces dermatitis, Coccidioides immitis, Histoplasma capsulatum) can produce spinal infections (5-10%) in healthy persons.200,201Fungi such as Coccidioides immitis and Blastomyces dermatitidis are limited to specific geographical areas whereas Candidaand Aspergillus are two organisms that most commonly cause fungal infections of the spine worldwide.202–204 Candida and asperigillus are normal commensals of the body and produce disease in susceptible organisms when they gain access to the vascular system through intravenous lines, during implantation of prosthetic devices or during surgery.201 For the other fungi, spinal involvement usually is the result of hematogenous or direct spread of organisms from an initial pulmonary source of infection.201 Vertebral body involvement can lead to vertebral compression fractures and gross deformities of the spine. Infection can spread along the anterior longitudinal ligament and can lead to psoas or paravertebral abscesses. Fungal infections may produce inflammatory reactions ranging from chronic inflammation to suppuration with granuloma formation.201,205
Early recognition of such conditions requires a high index of suspicion, a proper travel history and a detailed physical examination. Treatment for these fungi involves the use of prompt and appropriate antifungals and constant monitoring of clinical progress. Surgery is necessitated when resistance to therapy, spinal instability and neurologic deficits are indicated. Anterior debridement and stabilization with spinal fusion are preferred in such contexts.206,207 Prognosis varies, and it depends on the premorbid state of the patient, the type of organism and the timing of treatment. The unique characteristics of the fungi causing vertebral osteomyelitis are discussed in the sections below.
Coccidioidomycosis
Coccidioidomycosis is caused by the dimorphic fungi, Coccidioides immitis or Coccidioides posadasii. These organisms are endemic to certain arid regions of the western hemisphere. Inhalation is the most common route of infection, and primary infection usually goes unrecognized.208,209 Infection can disseminate beyond the lungs and the risk of this dissemination is estimated to be approximately 4.7 percent of recognized infections.210 When disseminated infection occurs, it usually is evident within weeks after initial exposure. Extrapulmonary dissemination is normally a result of hematogenous spread of the organism. Vertebrae are a common site for coccidioidal osteomyelitis with lesions present as single or multiples with occasional involvement of the sacrum and ileum.
The management of these lesions is especially important because of their potential impact on the spinal cord and mobility. Some may require surgical debridement and stabilization in order to control the infection. Identifying which lesions will benefit from surgery is challenging, and practices among different physicians vary significantly in management of these lesions. There are a couple of useful ways to image such infections. Radionuclide bone scans are useful to identify possible sites of infection.211 MRI with gadolinium enhancement has also been noted to be useful in identifying the extent of individual lesions.23
Upon completion of these studies and neurologic evaluation, patients should be managed depending on whether or not there is risk of or immediate cord compression and instability of the spinal column. For patients with cord compression, surgical decompression is warranted. For patients without cord compression and spinal instability, antifungal therapy may be initiated without immediate surgical intervention. Subsequent management depends upon the evolution of lesions as evidenced by neurologic exam and repeated MRI (within one or two months). If lesions are progressive, surgery can be reconsidered.
The drug of choice for treating such infections is a oral azole antifungal agent. Amphotericin B is usually implemented in patients with lesions that are worsening rapidly or are located in the spine. Surgical debridement or stabilization is critical as an adjunctive treatment in certain cases. Of note, for those patients who positively respond to antifungals, there is a high chance for relapse once treatment stops. The management of coccidioidomycosis in immunocompromised hosts who may require indefinite therapy presents additional challenges.
Blastomycosis
Blastomycosis is a systemic pyogranulomatous infection that primarily involves the lungs and arises after inhalation of the spore of Blastomyces dermatitidis. Hematogenous spread frequently occurs and can involve almost any organ. North America has reported the majority of cases of blastomycosis. Areas that can be considered at risk include the southeastern and south-central states bordering the Mississippi and Ohio River basins.212 Blastomycosis has been additionally reported in Africa, South America and Asia.
Extrapulmonary disease is usually seen in conjunction with active pulmonary infection. Among other sites, CNS and vertebral involvement are possible in patients infected with Blastomycosis. Though uncommon, CNS infection is possible and may present as meningitis, epidural abscess or intracranial abscesses.213 Osteomyelitis is among the most common extrapulmonary manifestations of blastomycosis after cutaneous involvement, and it occurs in up to 25 percent of patients with multiorgan disease.214 Any bone may be involved, but the most common are the vertebrae, pelvis and sacrum. Patients often present with soft tissue swelling or chronic draining sinus tract adjacent to the lesion of osteomyelitis, and there is usually little bone pain associated.212
Like many other infections, vertebral disease can be similar radiographically to tuberculosis, with anterior involvement of the vertebral body and destruction of the disc space.30 Additionally, paravertebral abscesses occur with vertebral blastomycosis.215 Diagnosis requires growth of the organism from a clinical specimen.212
Treatment of disseminated blastomycosis is determined by the presence or absence of central nervous system infection. Amphoceterin B followed by oral itraconazole for 12 months has been shown to be effective in treating moderately severe to severe disseminated disease.212 Bone disease is harder to treat and more likely to relapse than other forms of blastomycosis.212,216 Immunocompromised patients with blastomycosis should be treated with a lipid form of amphotericin B or amphotericin B deoxycholate intravenously for one to two weeks until improvement is observed.212 For patients with relapse in which immunosuppression cannot be reversed, itraconazole is recommended as a long term therapy.212
Candidiasis
Candidiasis is typically a fungal infection of the skin or mucous membrane. Candidemia is rare in immunocompetent hosts. Causative organisms of infection include C. albicans, C. tropicalis, and C. glabrata. C. albicans. Typically, infection due to candida species occurs in the thoracic and lumbar spine. Very rarely cases have been reported in the cervical spine. Risk factors include central venous catheters, immunosuppressive chemotherapy and use of broad spectrum antibiotics.
Presentation of candida vertebral osteomyelitis includes back pain, fever and neurological deficits; however, fever and neurological deficits are not as common. Symptoms are present for greater than one month in 83% of patients.47 Laboratory values such as white blood cell count are often normal however ESR is commonly elevated.47 Diagnostic tests include MRI, CT guided biopsy and culture. If suspected, early MRI will aid in detecting pathologic lesions with the diagnosis being confirmed with cultures from a CT guided biopsy.
The treatment for candida spinal infections varies. If the infection is caught early enough, antifungal treatment can be sufficient to treat and cure the patient. Amphotericin B and azoles are widely used. In more progressive cases, disease can lead to vertebral destruction and compression of neural elements. In these cases debridement, fusion and stabilization combined with antifungal agents can eradicate infection.217
Aspergillosis
Invasive aspergillosis can cause aspergillus osteomyelitis in sites such as the vertebrae, ribs and cranium among in patients. The most common species are A. fumigatus and A. flavus. Affected patients experience back pain and neurologic deficits. Risk factors that predispose patients to contracting this infection include corticosteroid therapy, widespread use of antibiotics and chemotherapeutic drugs.218 Along with this, primary immunodeficiency, solid organ transplant, IV drug use, COPD and diabetes mellitus have also been shown to increase risk.32
Aspergillus osteomyelitis is typically diagnosed by open biopsy and percutaneous biopsy. From this, organisms are obtained and revealed through direct culture or histology. MRI aids in looking for osteolysis, bone destruction and bone erosion.32 The most common feature of MRI include decreased signal intensity on T1-weighted images, increased signal on T2-weighted images and increased gadolinium contrast enhancement of T1-weighted images.32 Along with this, laboratory values of ESR and CRP are usually elevated.
Treatment consists of antifungal therapy and possibly surgical debridement. Acceptable antifungal agents include amphotericin B, itraconazole and voriconazole. As with candidiasis, spinal column instability or neural element compression is an indication for surgical intervention.
SECTION II: POSTOPERATIVE SPINAL INFECTIONS
Introduction
Postoperative spinal infections are a dreaded complication for both patient and physician. Despite advances in prophylactic antibiotics, surgical technique, sterilization technique, and pre and postoperative care surgical site infections continue to occur. As spinal surgery continues to become more sophisticated the number of invasive procedures, whether diagnostic or therapeutic, will certainly increase. Infection rates vary depending on duration and invasiveness of surgery, which calls for a deeper comprehension of the root causes for surgical site infections. It is imperative that surgeons understand the pathogenesis, risk factors, treatment and prevention as surgical site infection can have a negative effect on the overall outcome and satisfaction of the patient.
Epidemiology
The incidence of surgical site infections varies depending on certain variables such as invasiveness of surgery, operative time and use of instrumentation. Overall, spinal surgery has a higher rate of infection compared to orthopedic procedures such as hip and knee arthroplasties.219 Incidence rates for spinal infection following surgery of .7% to 16% have been reported.220–226
A study performed by Veeravagu et. al of 24, 774 patients who underwent spinal decompression and fusion showed 3.04% of patients had a postoperative infection; 1.16% were superficial and 1.89% were deep infections.221 Other studies show an incidence rate of .6% to 5% after discectomy. Fusion without instrumentation has shown incidence rates of .4% to 4.3%. When looking at the use of internal fixation, this increases the rate of infection to 6.6 to 8.7%.227,228
Infection rates are directly correlated with more complex surgeries; however, the exact reason is not clearly defined in the literature. It has been postulated that the increased amounts of soft tissue dissection allow for more bacterial colonization.229 Instrumentation also increases the risk of infection in patients. Micromotion of the instrumentation leads to granuloma formation and provides yet another medium for bacterial colonization.230 Operative time also plays a role in the incidence of surgical site infections. It has been shown that there is increased risk with procedures lasting longer than 3 hours, although this risk may be related to anesthesia risk.231 Meticulous preoperative planning and sound execution can help lower incidence rates among patients following spinal procedures.
Pathogenesis
Bacteria must be present at the surgical site in large quantities (greater than 105 organisms) in order for a clinical infection to occur.229 Bacteria may be introduced through direct inoculation from the patient’s skin, hematogenous spread from a distant source in the patient’s body, or soiling of the incision in the fresh postoperative phase.232 Most infections however are a result of direct inoculation at the time of surgery.
Gram positive cocci are the primary cause of surgical site infections of the spine. Staphylococcus aureus, Staphylococcus epidermidis and β hemolytic Streptococci are the main bacteria that are found in cases of postoperative spinal infection. Of these, S. aureus accounts for up to 60% of surgical site infections.233 Following this, gram negative bacteria are the next most common bacterial causes of infection. These include Klebsiella pneumoniae, Escheria coli, Pseudomonas aeruginosa and the proteus species. Patients with a known history of drug abuse are at higher incidence of infection with gram negative rods.228 Propionibacterium acnes and diptheroids are also known causes of chronic or delayed infections.220,234 These are commonly found in the microflora of the skin leading some to believe that prolonged postoperative drainage is a risk factor in the development of delayed postoperative spine infections.235 In a study by Koutsoumbelis et. al, 34% of positive cultures revealed methicillin resistant staphylococcus aureus.236 Incidence rates with MRSA as the concomitant organism can be correlated with risk factors such as previous hospitalization, intensive care unit stay, indwelling catheters, prolonged antibiotic therapy, advanced age and exposure to patients colonized or infected with MRSA.237
Risk factors
Modifiable and non-modifiable risk factors have been shown to correlate with surgical site spinal infections. Some of these risk factors include insulin dependent diabetes,238 history of weight loss before surgery,239 disseminated malignance or operating through irradiated skin,221,233 chronic steroid use,240 anemia,221,241 obesity, tobacco use,239 and recent epidural steroid injections.242–245 The presence of instrumentation and a history of smoking have also been shown to increase risk of infection. Longer postoperative stays have been shown to increase infection rates by as much as 50%.221
Operation and disease specific risk factors also carry the potential to increase the incidence of surgical site infections. Among these are operations involving the sacrum, long fusion constructs,240 operative time greater than three hours,221 revision surgery,246 intraoperative dural tear247 and neuromuscular disease. Other technical factors associated with higher infection rates include inattention to aseptic technique, crowding and excessive movement in the operation room, and colonization of the members of the operating team.248 Careful perioperative planning can keep modifiable risk factors to a minimum and lead to lower infection rates.
Along with the aforementioned, recent epidural steroid injections have been shown to increase the chances of contracting postoperative spinal infections.244,249It was found that timing of epidural steroid injections plays a role in the risk of attaining a postoperative infection. In a retrospective database analysis performed by Cancienne et al., 300,000 patients were divided into four cohorts to assess risk of infection post epidural steroid injection. These consisted of a control group of patients who underwent posterior cervical fusion (PCF) or anterior cervical discectomy and fusion (ACDF) without prior cervical epidural steroid injection (CESI) and three cohorts who underwent PCF or ACDF with prior CESI within 3 months, 3 to 6 months and 6 to 12 months. It was concluded that patients who underwent CESI within 6 months of PCF had significantly increased rates of postoperative infection. Along with this, patients who underwent CESI within 3 months of ACDF were shown to be independently associated with significantly increased rates of postoperative infection.250
Prevention
Preoperatively prophylactic antibiotics have proven effective in minimizing surgical site infections. First generation cephalosporins are typically used and most effective in preventing a postoperative infection from occurring. Other antibiotics, if the patient is allergic to penicillins, have proven effective including vancomycin, clindamycin and ciprofloxacin.220,221,245,251,252 In a study by Keller and Pappas, infection rates have decreased from 2.7% to 0% as a result of prophylactic antibiotics.253 In another study, postoperative infections have decreased from 9.3% to 1% after lumbar diskectomies with the use of prophylactic antibiotics.
Povidoneiodine (Betadine) is a complex of polyvinyl pyrrilidine and triiodine ions that is widely used as an antiseptic for skin, mucous membranes and wounds. Povidoneiodine has bactericidal activity against a wide spectrum of pathogens, including methicillin-resistant Staphylococcus aureus (MRSA).254–259 In a study by Cheng et. al. a population of four hundred and fourteen patients were randomized to two groups. In group one, 218 patients’ surgical wounds were irrigated with dilute 3.5% betadine solution. In group two, consisting of 206 patients, betadine irrigation was not performed. Perioperative management remained constant between the groups. At a mean follow up time of 15.5 months, group one did not have any wound infections. Group two had one superficial infection (0.5%) and six deep infections (2.9%). The difference between the groups was statistically significant.260 Taking into account the effectiveness of prophylactic antibiotics and betadine irrigation, a combination of the two may aid in decreasing the incidence of surgical site infections.
Intraoperative instilment of vancomycin powder has become widely popular among spinal surgeries as a means of preventing infection. In combination with prophylactic antibiotics, this has proven effective in decreasing the incidence and preventing surgical site infections. There are many benefits to the use of intraoperative vancomycin powder instillation such as the effective broad coverage against organisms including MRSA, ease of use and cost-effectiveness.261 Vancomycin powder has also been shown to have poor systemic absorption allowing it to act better locally to prevent infection.262 In a study performed by Molinari et. al, 1,512 patients who underwent spinal surgery had a 0.99% incidence rate of surgical site infections.263 In another study wound infection rates were decreased from 13% to 0% with the application of 1g of vancomycin powder in 110 patients after posterior spinal fusion.264 According to O’Neil et. al, in a study of 171 patients who underwent posterior cervical fusion, infection rates declined from 10.9% to 2.5% with application of 1g of vancomycin powder.264
Clinical presentation
Postoperative surgical site infections may present as a superficial or deep infection. Superficial infections involve only the dermis and subcutaneous layer. Deep infections occur beneath the lumbodorsal fascia. Superficial infections often present within the first two weeks postoperatively. Commons symptoms include tenderness to palpation, erythema, edema, drainage, low grade fever and elevated ESR levels.235
Deep infections are often delayed and cause patients greater pain than superficial infections and are generally associated with malaise, and in some cases anorexia. CRP and ESR are increased to a greater magnitude when compared to superficial infections. However, a normal white cell count and the absence of fever does exclude a deep surgical site infection.235 The presence of a neurological deficit should raise suspicion for an epidural abscess. Of epidural abscesses, 16% occur in the postoperative period.228 Significant pain with lumbar range of motion is a common finding for postoperative diskitis.227
Severe surgical site infections may cause hypotension, lethargy, confusion and other signs of sepsis. Sepsis or progressive neurologic deficits are absolute indications for emergent irrigation and debridement however they are uncommon findings.235,229
Treatment
Treatment is selected dependent upon the type of surgical site infection as well as the timing in regards to when the infection is detected. CT guided biopsy or blood cultures may be helpful to identify the pathogen causing the infection. However, CT aspiration is only about 39% sensitive.265 Administration of antibiotics in a timely manner is essential to patient outcome. Conservative and surgical management both have roles in treating postoperative spinal infections. Treatment ranges from antibiotics, wound explorations, irrigation and debridement with or without instrumentation removal, and closed suction irrigation systems.
In patients undergoing conservative treatment, intravenous culture specific antibiotics should be administered for approximately 6 weeks; however, the exact length of antibiotics should be directly related to patient response. Serial clinical examination and laboratory surveillance tests with WBC count, ESR and CRP should be managed.229 In cases where antibiotic resistant organisms are involved, new classes of antibiotics such as quinupristin/dalfopristin and linezolid have proven effective against 80% of vancomycin resistant enterococci and gram positive infections.237 Along with this, wound management can also be effective in eradicating surgical site infections. If there is no improvement with antibiotic treatment or progression of symptoms, wound exploration and surgical irrigation and debridement should be considered.
Epidural abscesses may be treated with antibiotics if small in size and in the lumbar region; however, if presenting with worsening neurological symptoms, this would be an indication for surgical decompression. The surgical approach, whether anterior or posterior depends on the location of the abscess in relation to the spinal canal. Anterior abscesses may necessitate vertebral debridement, anterior graft placement and also posterior reconstruction. Long segment abscesses that are still liquid can be treated by a laminotomy at the inferior end of the abscess and irrigation of the epidural space with a fine silicon catheter.248
Postoperative spondylodisciitis is the most resilient surgical site infection as antibiotic therapy has poor osseous penetration. This requires long durations of antibiotic therapy with some patients being successfully treated without surgical debridement.266 Typically, culture specific antibiotics are given intravenously for six weeks and orally for the next six weeks. Most organisms are sensitive to cephalosporins. However, MRSA and other resistant organisms would require antibiotics like vancomycin, linezolid, piperacillin, and clindamycin.248 If there is no improvement with antibiotics or there is worsening of neurologic symptoms, operative intervention must be considered.
At the time of surgical debridement of a postoperative infection, instrumentation that is stable is retained if diagnosed in the early postoperative period. In one study,224 132 postoperative spinal infections diagnosed early retained stable and replaced loose implants at debridement and reported that 76% of postoperative infections were treated successfully with only 1 debridement. A study done by Glassman, 19 instrumented patients who developed a deep infection and were treated by repeat debridement (average 4.7 procedures) successfully retained their implants and were disease free at 1 year follow up.267 Mok et al. states that implant preservation can be done with eradication of infection in most cases with early diagnosis, aggressive debridement and proper antibiotic therapy.268 There are risks of non-union and loss of correction of deformity as a result of removal of instrumentation. However, in delayed infections, instrumentation should be removed.236
Conclusion
Understanding the risk factors for a postoperative spinal infection can aid in its prevention. When possible, modifiable risk factors should be changed. Early recognition and diagnosis is the key in successfully eradicating a postoperative infection.
REFERENCES
- A controlled trial of ambulant out-patient treatment and in-patient rest in bed in the management of tuberculosis of the spine in young Korean patients on standard chemotherapy a study in Masan, Korea. First report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br. 1973;55(4):678-697.
- Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis. 1979;1(5):754-776.
- Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis. 2006;38(8):728-730.
- Sethna NF, Clendenin D, Athiraman U, Solodiuk J, Rodriguez DP, Zurakowski D. Incidence of epidural catheter-associated infections after continuous epidural analgesia in children. Anesthesiology. 2010;113(1):224-232.
- Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br. 1979;61(1):47-54.
- Frederickson B, Yuan H, Olans R. Management and outcome of pyogenic vertebral osteomyelitis. Clin Orthop Relat Res. 1978;(131):160-167.
- Griffiths HE, Jones DM. Pyogenic infection of the spine. A review of twenty-eight cases. J Bone Jointt Surg Br. 1971;53(3):383-391.
- Kemp HB, Jackson JW, Jeremiah JD, Hall AJ. Pyogenic infections occurring primarily in intervertebral discs. J Bone Joint Surg Br. 1973;55(4):698-714.
- Kulowski J. Pyogenic osteomyelitis of the spine. J Bone Joint Surg Am. 1936;18(2):343-364.
- Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970;282(4):198-206. doi:10.1056/nejm197002052820606.
- Waldvogel FA, Papageorgiou PS. Osteomyelitis: the past decade. N Engl J Med. 1980;303(7):360-370.
- Solera J, Lozano E, Martinez-Alfaro E, Espinosa A, Castillejos ML, Abad L. Spondylitis: review of 35 cases and literature survey. Clin Infect Dis. 1999;29(6):1440-1449.
- McDonald M. Vertebral Osteomyelitis and Discitis in Adults. UpToDate. 2016. Available at https://www.uptodate.com/contents/vertebral-osteomyelitis-and-discitis-in-adults. Accessed on May 19, 2019.
- Beronius M, Bergman B Andersson R. Vertebral osteomyelitis in Goteborg, Sweden: a retrospective study of patients during 1990-95. Scand J Infect Dis. 2001;33(7):527-532.
- Gupta A, Kowalski TJ, Osmon DR, et al. Long-term outcome of pyogenic vertebral osteomyelitis: a cohort study of 260 patients. Open Forum Infect Dis. 2014;1(3):ofu107.
- Pigrau C, Rodriguez-Pardo D, Fernandez-Hidalgo N, et al. Health care associated hematogenous pyogenic vertebral osteomyelitis: a severe and potentially preventable infectious disease. Medicine (Baltimore). 2015;94(3):e365.
- Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med. 2008;168(8):805-819.
- Vartzelis G, Theodoridou M, Daikos GL, Dellagrammaticas H, Syriopoulou VP. Brain abscesses complicating Staphylococcus aureus sepsis in a premature infant. Infection. 2005;33(1):36-38.
- Baker AS, Ojemann RG, Swartz MN, Richardson EP Jr. Spinal Epidural Abscess. N Engl J Med. 1975;293(10):463-468.
- Graybill JR, Sobel J, Saag M, et al. Diagnosis and management of increased intracranial pressure in patients with aids and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis. 2000;30(1):47-54. doi:10.1086/313603.
- Hodgson AR. Report on the findings and results in 300 cases of Pott’s disease treated by anterior fusion of the spine. J West Pacific Orthop Assoc. 1964;1:3.
- Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):e26-46.
- Erly WK, Carmody RF, Seeger JF, Lund PJ. Magnetic resonance imaging of coccidioidal spondylitis. Int J Neuroradiol. 1997;3(5):385-392.
- Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19(2):259-282.
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016:62(4):e1-50.
- Kapeller P, Fazekas F, Krametter D, et al. Pyogenic infectious spondylitis: clinical, laboratory and MRI features. Eur Neurol. 1997;38(2):94-98.
- Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291.
- Tuchman A, Pham M, Hsieh PC. The indications and timing for operative management of spinal epidural abscess: literature review and treatment algorithm. Neurosurg Focus. 2014;37(2):E8.
- Controlled trial of short-course regimens of chemotherapy in the ambulatory treatment of spinal tuberculosis. Results at three years of a study in Korea. Twelfth report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br. 1993;75(2):240-248.
- Guler N, Palanduz A, Ones U, et al. Progressive vertebral blastomycosis mimicking tuberculosis. Pediatr Infect Dis J. 1995;14(9):816-818.
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21(1):28-34.
- Gamaletsou MN, Rammaert B, Bueno MA, et al. Aspergillus osteomyelitis: epidemiology, clinical manifestations, management, and outcome. J Infect. 2014;68(5):478-493. doi:10.1016/j.jinf.2013.12.008.
- Ross PM, Fleming JL. Vertebral Body Osteomyelitis: spectrum and natural history. A retrospective analysis of 37 cases. Clin Orthop Relat Res. 1976;(118):190-198.
- Batson OV. The vertebral system of veins as a means for cancer dissemination. Prog Clin Cancer. 1967;3:1-18.
- Cahill DW, Love LC, Rechtine GR. Pyogenic osteomyelitis of the spine in the elderly. J Neurosurg. 1991;74(6):878-886.
- Wiley AM, Trueta J. The vascular anatomy of the spine and its relationship to pyogenic vertebral osteomyelitis. J Bone Joint Surg Br. 1959;41-B:796-809.
- Sapico FL, Montgomerie JZ. Vertebral osteomyelitis. Infect Dis Clin North Am. 1990;4(3):539-550.
- Larson DL, Hudak KA, Waring WP, Orr MR, Simonelic K. Protocol management of late-stage pressure ulcers: a 5-year retrospective study of 101 consecutive patients with 179 ulcers. Plast Reconstr Surg. 2012;129(4):897-904.
- Türk EE, Tsokos M, Delling G. Autopsy-based assessment of extent and type of osteomyelitis in advanced-grade sacral decubitus ulcers: a histopathologic study. Arch Pathol Lab Med. 2003;127(12):1599-1602.
- Park KH, Cho OH, Jung M, et al. Clinical characteristics and outcomes of hematogenous vertebral osteomyelitis caused by gram-negative bacteria. J Infect. 2014;69(1):42-50. doi:10.1016/j.jinf.2014.02.009.
- Michel-Batot C, Dintinger H, Blum A, et al. A particular form of septic arthritis: septic arthritis of facet joint. Joint Bone Spine. 2008;75(1):78-83.
- Danner RL, Hartman BJ. Update of spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis. 1987;9(2):265-274.
- Dunphy L, Iyer S, Brown C. Rare cause of back pain: Staphylococcus aureus vertebral osteomyelitis complicated by recurrent epidural abscess and severe sepsis. BMJ Case Rep. 2016;2016.
- Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369-379. doi:10.1016/s0140-6736(04)16727-5.
- Marschall J, Bhavan KP, Olsen MA, Fraser VJ, Wright NM, Warren DK. The impact of prebiopsy antibiotics on pathogen recovery in hematogenous vertebral osteomyelitis. Clin Infect Dis. 2011;52(7):867-872.
- Patzakis MJ, Rao S, Wilkins J, Moore TM, Harvey PJ. Analysis of 61 cases of vertebral osteomyelitis. Clin Orthop Relat Res. 1991;(264):178-183.
- Miller DJ, Mejicano GC. Vertebral osteomyelitis due to Candida species: case report and literature review. Clin Infect Dis. 2001;33(4):523-530.
- Hendrickx L, Van Wijngaerden E, Samson I, Peetermans WE. Candidal vertebral osteomyelitis: report of 6 patients, and a review. Clin Infect Dis. 2001;32(4):527-533.
- Kauffman CA. Candida Osteoarticular Infections. In: UpToDate. (Post T, ed.). Waltham: UpToDate; 2016.
- Fischman AJ, Alpert NM, Livni E, et al. Pharmacokinetics of 18F-labeled fluconazole in healthy human subjects by positron emission tomography. Antimicrob Agents Chemother. 1993;37(6):1270-1277.
- Ramamohan N, Zeineh N, Grigoris P, Butcher I. Candida glabrata infection after total hip arthroplasty. J Infect. 2001;42(1):74-76.
- Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol. 1999;18(5):406-409.
- Günthard HF, Meltzer H, Karpinski NC, Ziegler EJ. Spinal osteomyelitis caused by Candida glabrata: case report and review. Infect Dis Clin Pract. 1998;7(2):112-116.
- Yang SC, Shao PL, Hsueh PR, Lin KH, Huang LM. Successful treatment of Candida tropicalis arthritis, osteomyelitis and costochondritis with caspofungin and fluconazole in a recipient of bone marrow transplantation. Acta Paediatr. 2006;95(5):629-630.
- Peman J, Jarque I, Bosch M, et al. Spondylodiscitis caused by Candida krusei: case report and susceptibility patterns. J Clin Microbiol. 2006;44(5):1912-1914.
- Gamaletsou MN, Kontoyiannis DP, Sipsas N V, et al. Candida osteomyelitis: analysis of 207 pediatric and adult cases (1970-2011). Clin Infect Dis. 2012;55(10):1338-1351. doi:10.1093/cid/cis660.
- Carek PJ, Dickerson LM, Sack JL. Diagnosis and management of osteomyelitis. Am Fam Physician. 2001;63(12):2413-2421.
- Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385(9971):875-882.
- Park KH, Cho OH, Lee JH, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis. 2016;62(10):1262-1269.
- McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long term outcome for 253 patients from 7 cleveland area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
- Sexton DJ, Sampson JH. Spinal epidural abscess. UpToDate. 2016. Available at https://www.uptodate.com/contents/spinal-epidural-abscess. Accessed on May 18, 2019.
- Salkind AR, Wormser GP. Infections of the Central Nervous System, 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:928.
- Bond A, Manian FA. Spinal epidural abscess: a review with special emphasis on earlier diagnosis. Biomed Res Int. 2016;2016:1614328.
- Sendi P, Bregenzer T, Zimmerli W. Spinal epidural abscess in clinical practice. QJM. 2008;101(1):1-12.
- Sorensen P. Spinal epidural abscesses: conservative treatment for selected subgroups of patients. Br J Neurosurg. 2003;17(6):513-518.
- Ptaszynski AE, Hooten WM, Huntoon MA. The incidence of spontaneous epidural abscess in olmsted county from 1990 through 2000: a rare cause of spinal pain. Pain Med. 2007;8(4):338-343.
- Darouiche RO. Spinal Epidural Abscess. N Engl J Med. 2006;355(19):2012-2020.
- Krishnamohan P, Berger JR. Spinal Epidural Abscess. Curr Infect Dis Rep. 2014;16(11). 436.
- Cook TM, Counsell D, Wildsmith JAW. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102(2):179-190.
- Popping DM, Zahn PK, Van Aken HK, Dasch B, Boche R, Pogatzki-Zahn E. Effectiveness and safety of postoperative pain management: a survey of 18 925 consecutive patients between 1998 and 2006 (2nd revision): a database analysis of prospectively raised data. Br J Anaesth. 2008;101(6):832-840.
- Reynolds F. Neurological infections after neuraxial anesthesia. Anesthesiol Clin. 2008;26(1):23-52.
- Scott DB, Hibbard BM. Serious non-fatal complications associated with extradural block in obstetric practice. Br J Anaesth. 1990;64(5):537-541.
- Nussbaum ES, Rigamonti D, Standiford H, Numaguchi Y, Wolf AL, Robinson WL. Spinal epidural abscess: a report of 40 cases and review. Surg Neurol. 1992;38(3):225-231.
- Darouiche RO, Hamill RJ, Greenberg SB, Weathers SW, Musher DM. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore). 1992;71(6):369-385.
- Rigamonti D, Liem L, Wolf AL, et al. Epidural abscess in the cervical spine. Mt Sinai J Med. 1994;61(4):357-362.
- Griffiths DL. Tuberculosis of the spine: a review. Adv Tuberc Res. 1979;20:92-110.
- Holt HM, Andersen SS, Andersen O, Gahrn-Hansen B, Siboni K. Infections following epidural catheterization. J Hosp Infect. 1995;30(4):253-260.
- Phillips JM, Stedeford JC, Hartsilver E, Roberts C. Epidural abscess complicating insertion of epidural catheters. Br J Anaesth. 2002;89(5):778-782.
- Torda AJ, Gottlieb T, Bradbury R. Pyogenic vertebral osteomyelitis: analysis of 20 cases and review. Clin Infect Dis. 1995;20(2):320-328.
- Ju KL, Kim S Do, Melikian R, Bono CM, Harris MB. Predicting patients with concurrent noncontiguous spinal epidural abscess lesions. Spine J. 2015;15(1):95-101.
- Chen WC, Wang JL, Wang JT, Chen YC, Chang SC. Spinal epidural abscess due to Staphylococcus aureus: clinical manifestations and outcomes. J Microbiol Immunol Infect. 2008;41(3):215-221.
- Curry WT Jr, Hoh BL, Amin-Hanjani S, Eskandar EN. Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol. 2005;63(4):364-371.
- Davis DP, Salazar A, Chan TC, Vilke GM. Prospective evaluation of a clinical decision guideline to diagnose spinal epidural abscess in patients who present to the emergency department with spine pain. J Neurosurg Spine. 2011;14(6):765-770.
- Wong D, Raymond NJ. Spinal epidural abscess. N Z Med J. 1998;111(1073):345-347.
- de Lucas EM, González Mandly A, Gutiérrez A, et al. CT-guided fine-needle aspiration in vertebral osteomyelitis: true usefulness of a common practice. Clin Rheumatol. 2009;28(3):315-320.
- Euba G, Narvaez JA, Nolla JM, et al. Long-term clinical and radiological magnetic resonance imaging outcome of abscess-associated spontaneous pyogenic vertebral osteomyelitis under conservative management. Semin Arthritis Rheum. 2008;38(1):28-40.
- Kricun R, Shoemaker EI, Chovanes GI, Stephens HW. Epidural abscess of the cervical spine: MR findings in five cases. AJR Am J Roentgenol. 1992;158(5):1145-1149.
- Hancock DO. A study of 49 patients with acute spinal extradural abscess. Paraplegia. 1973;10(4):285-288.
- Angtuaco EJ, McConnell, Chadduck WM, Flanigan S. MR imaging of spinal epidural sepsis. AJR Am J Roentgenol. 1987;149(6):1249-1253.
- Post MJ, Sze G, Quencer RM, Eismont FJ, Green BA, Gahbauer H. Gadolinium-enhanced MR in spinal infection. J Comput Assist Tomogr. 1990;14(5):721-729.
- Rigamonti D, Liem L, Sampath P, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52(2):189-197.
- Parkinson JF, Sekhon LH. Spinal epidural abscess: appearance on magnetic resonance imaging as a guide to surgical management. Report of five cases. Neurosurg Focus. 2004;17(6):E12
- Uchida K, Nakajima H, Yayama T, et al. Epidural abscess associated with pyogenic spondylodiscitis of the lumbar spine; evaluation of a new MRI staging classification and imaging findings as indicators of surgical management: a retrospective study of 37 patients. Arch Orthop Trauma Surg. 2010;130(1):111-118.
- Liem LK, Rigamonti D, Wolf AL, Robinson WL, Edwards CC, DiPatri A. Thoracic epidural abscess. J Spinal Disord. 1994;7(5):449-454.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267-1284.
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55.
- Pfausler B, Spiss H, Beer R, et al. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J Neurosurg. 2003;98(5):1040-1044.
- Nau R, Prange HW, Menck S, Kolenda H, Visser K, Seydel JK. Penetration of rifampicin into the cerebrospinal fluid of adults with uninflamed meninges. J Antimicrob Chemother. 1992;29(6):719-724.
- Darouiche RO, Hamill RJ, Greenberg SB, Weathers SW, Musher DM. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore). 1992;71(6):369-385.
- Firsching R, Frowein RA, Nittner K. Acute spinal epidural empyema. Observations from seven cases. Acta Neurochir (Wien). 1985;74(1-2):68-71.
- Rea GL, McGregor JM, Miller CA, Miner ME. Surgical treatment of the spontaneous spinal epidural abscess. Surg Neurol. 1992;37(4):274-279.
- Adogwa O, Karikari IO, Carr KR, et al. Spontaneous spinal epidural abscess in patients 50 years of age and older: a 15-year institutional perspective and review of the literature. J Neurosurg Spine. 2014;20(3):344-349.
- Connor DE Jr, Chittiboina P, Caldito G, Nanda A. Comparison of operative and nonoperative management of spinal epidural abscess: a retrospective review of clinical and laboratory predictors of neurological outcome. J Neurosurg Spine. 2013;19(1):119-127.
- Doutchi M, Seng P, Menard A, et al. Changing trends in the epidemiology of vertebral osteomyelitis in Marseille, France. New Microbes New Infect. 2015;7:1-7.
- Schoenfeld AJ, Wahlquist TC. Mortality, complication risk, and total charges after the treatment of epidural abscess. Spine J. 2015;15(2):249-255.
- Shiban E, Janssen I, Wostrack M, et al. A retrospective study of 113 consecutive cases of surgically treated spondylodiscitis patients. A single-center experience. Acta Neurochir (Wien). 2014;156(6):1189-1196.
- Shweikeh F, Saeed K, Bukavina L, Zyck S, Drazin D, Steinmetz MP. An institutional series and contemporary review of bacterial spinal epidural abscess: current status and future directions. Neurosurg Focus. 2014;37(2):E9.
- Bonfiglio M, Lange TA, Kim YM. Pyogenic Vertebral Osteomyelitis. Disk Space infections. Clin Orthop Relat Res. 1973;(96):234-247.
- Khanna RK, Malik GM, Rock JP, Rosenblum ML. Spinal epidural abscess: evaluation of factors influencing outcome. Neurosurgery. 1996;39(5):958-964.
- Bynum H. Spitting Blood: The History of Tuberculosis. Oxford University Press; 2012.
- Watts HG, Lifeso RM. Tuberculosis of bones and joints. J Bone Joint Surg Am. 1996;78(2):288-298.
- Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120(4):316-353.
- Teo HE, Peh WC. Skeletal tuberculosis in children. Pediatr Radiol. 2004;34(11):853-860.
- Fanning A. Tuberculosis: 6. extrapulmonary disease. CMAJ. 1999;160(11):1597-1603.
- Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49(9):1350-1357.
- Vohra R, Kang HS, Dogra S, Saggar RR, Sharma R. Tuberculous osteomyelitis. J Bone Joint Surg. 1997;79(4):562-566.
- Collert S. Osteomyelitis of the Spine. Acta Orthop Scand. 1977;48(3):283-290.
- Adams ZB. Tuberculosis of the Spine in Children. J Bone Joint Surg Am. 1940;22(3):860-861.
- Adendorff JJ, Boeke EJ, Lazarus C. Pott’s paraplegia. S Afr Med J. 1987;71(7):427-428.
- Martin NS. Pott’s paraplegia: a report of 120 cases. J Bone Joint Surg Br. 1971;53(B):596-608.
- Martin NS. Tuberculosis of the spine. A study of the results of treatment during the last twenty-five years. J Bone Joint Surg Br.1970;52(4):613-628.
- Bailey HL, Gabriel M, Hodgson AR, Shin JS. Tuberculosis of the Spine in Children. Operative findings in one hundred consecutive patients treated by removal of the lesion and anterior grafting. J Bone Joint Surg Am. 1972;54(8):1633-1657.
- Bosworth DM, Della Pietra A, Rahilly G. Paraplegia Resulting from Tuberculosis of the Spine. J Bone Joint Surg. 1953;35(3):735-740.
- Cleveland M. Tuberculosis of the spine. A clinical study of 203 patients from Sea View and St. Luke’s Hospital. Am Rev Tuberc. 1940;41:215-231.
- Dobson J. Tuberculosis of the spine; an analysis of the results of conservative treatment and of the factors influencing the prognosis. J Bone Joint Surg Br. 1951;33-B(4):517-531.
- Fellander M. Paraplegia in spondylitis: results of operative treatment. Paraplegia. 1975;13(2):75-88.
- Friedman B. Chemotherapy of Tuberculosis of the Spine. J Bone Joint Surg Am. 1966;48(3):451-474.
- Hodgson AR, Yau A, Kwon JS, Kim D. 12 A Clinical Study of 100 Consecutive Cases of Pott’s Paraplegia. Clin Orthop Relat Res. 1964;(36):128-150.
- Kaplan CJ. Pott’s disease in South African Bantu children; an analysis of results and comparison with Lancashire figures. Br J Tuberc Dis Chest. 1952;46(4):209-213.
- Adendorff JJ, Boeke EJ, Lazarus C. Tuberculosis of the spine: results of management of 300 patients. J R Coll Surg Edinb. 1987;32(3):152-155.
- Weinberg JA. The surgical excision of psoas abscesses resulting from spinal tuberculosis. J Bone Joint Surg Am. 1957;39-A(1):17-27.
- Lifeso RM, Weaver P, Harder EH. Tuberculous spondylitis in adults. J Bone Jt Surg Am. 1985;67(9):1405-1413.
- Five year assessments of controlled trials of ambulatory treatment, debridement and anterior spinal fusion in the management of tuberculosis of the spine. Studies in Bulawayo (Rhodesia) and in Hong Kong. Sixth report of the Medical Research Council Working Party on tuberculosis of the spine. J Bone Joint Surg Br. 1978;60B-(2):163-177.
- Moodie AS. Tuberculosis in Hong Kong. Tubercle. 1963;44(3):334-345.
- Compere EL, Garrison M. Correlation of Pathologic and Roentgenologic Findings in Tuberculosis and Pyogenic Infections of the Vertebrae. Ann Surg. 1936;104(6):1038-1067.
- La Rocca H. Spinal sepsis. In: Rothman RH, Simeone FA, eds. The Spine. Vol 2nd. Philadelphia: W.B. Saunders Co.; 1982:757-774.
- Tuli SM. Tuberculosis of the Spine: a historical review. Clin Orthop Relat Res. 2007;460:29-38.
- Fuentes Ferrer M, Gutierrez Torres L, Ayala Ramirez O, Rumayor Zarzuelo M, del Prado Gonzalez N. Tuberculosis of the spine. A systematic review of case series. Int Orthop. 2012;36(2):221-231.
- Sexton DJ, McDonald M. Skeletal Tuberculosis. UpToDate. 2016. Available at https://www.uptodate.com/contents/skeletal-tuberculosis. Accessed on May 20, 2019.
- Garg RK, Somvanshi DS. Spinal tuberculosis: a review. J Spinal Cord Med. 2011:34(5):440-454.
- Baaj AA, Mummaneni PV, Uribe JS, Vaccaro AR, Greenberg MS, Eds. Handbook of Spine Surgery. New York:Thieme Publishing Group; 2012.
- Kotil K, Alan MS, Bilge T. Medical management of Pott disease in the thoracic and lumbar spine: a prospective clinical study. J Neurosurg Spine. 2007;6(3):222-228.
- Weaver P, Lifeso RM. The radiological diagnosis of tuberculosis of the adult spine. Skeletal Radiol. 1984;12(3):178-186.
- Khoo LT, Mikawa K, Fessler RG. A surgical revisitation of Pott distemper of the spine. Spine J. 2003;3(2):130-145.
- Nussbaum ES, Rockswold GL, Bergman TA, Erickson DL, Seljeskog EL. Spinal tuberculosis: a diagnostic and management challenge. J Neurosurg. 1995;83(2):243-247.
- Yao DC, Sartoris DJ. Musculoskeletal tuberculosis. Radiol Clin North Am. 1995;33(4):679-689.
- Pertuiset E, Beaudreuil J, Liote F, et al. Spinal tuberculosis in adults. A study of 103 cases in a developed country, 1980-1994. Medicine (Baltimore). 1999;78(5):309-320.
- Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. ARJ Am J Roentgenol. 2004;182(6):1405-1410.
- Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147-e195.
- Blumberg HM, Leonard MK Jr. Jasmer RM. Update on the treatment of tuberculosis and latent tuberculosis infection. JAMA. 2005;293(22):2776-2784.
- Upadhyay SS, Orth D, Sell P, Saji MJ, Sell B, Hsu LC. Surgical management of spinal tuberculosis in adults: Hong Kong operation compared with debridement surgery for short and long term outcome of deformity. Clin Orthop Relat Res. 1994;302:173-182.
- Garg N, Vohra R. Minimally invasive surgical approaches in the management of tuberculosis of the thoracic and lumbar spine. Clin Orthop Relat Res. 2014;472(6):1855-1867.
- Chan ST, Leung S. Spinal epidural abscess following steroid injection for sciatica. Case Report. Spine (Phila Pa 1976). 1989;14(1):106-108.
- Hodgson AR, Stock FE, Fang HS, Ong GB. Anterior spinal fusion the operative approach and pathological findings in 412 patients with pott’s disease of the spine. Br J Surg. 1960;48(208):172-178.
- Benli IT, Kaya A, Acaroglu E. Anterior instrumentation in tuberculous spondylitis. Clin Orthop Relat Res. 2007;460:108-116.
- Jain AK, Dhammi IK, Prashad B, Sinha S, Mishra P. Simultaneous anterior decompression and posterior instrumentation of the tuberculous spine using an anterolateral extrapleural approach. J Bone Joint Surg Br. 2008;90(11):1477-1481.
- Kandwal P, G V, Jayaswal A. Management of tuberculous infection of the spine. Asian Spine J. 2016;10(4):792-800.
- Rajasekaran S. Kyphotic deformity in spinal tuberculosis and its management. Int Orthop. 2012;36(2):359-365.
- Singh S, Kumaraswamy V, Sharma N, Saraf SK, Khare GN. Evaluation of role of anterior debridement and decompression of spinal cord and instrumentation in treatment of tubercular spondylitis. Asian Spine J. 2012;6(3):183.
- Zhang HQ, Li JS, Zhao SS, et al. Surgical management for thoracic spinal tuberculosis in the elderly: posterior only versus combined posterior and anterior approaches. Arch Orthop Trauma Surg. 2012;132(12):1717-1723.
- Garg B, Kandwal P, Nagaraja UB, Goswami A, Jayaswal A. Anterior versus posterior procedure for surgical treatment of thoracolumbar tuberculosis: a retrospective analysis. Indian J Orthop. 2012;46(2):165-170.
- Rajasekaran S. The natural history of post-tubercular kyphosis in children. J Bone Joint Surg. 2001;83(7):954-962.
- Suk SI, Kim JH, Kim WJ, Lee SM, Chung ER, Nah KH. Posterior vertebral column resection for severe spinal deformities. Spine (Phila Pa 1976). 2002;27(21):2374-2382.
- Zhou T, Li C, Liu B, Tang X, Su Y, Xu Y. Analysis of 17 cases of posterior vertebral column resection in treating thoracolumbar spinal tuberculous angular kyphosis. J Orthop Surg Res. 2015;10:64.
- Deng Y, Lv G, An HS. En bloc spondylectomy for the treatment of spinal tuberculosis with fixed and sharply angulated kyphotic deformity. Spine (Phila Pa 1976). 2009;34(20):2140-2146.
- Wang Y, Zhang Y, Zhang X, et al. Posterior-only multilevel modified vertebral column resection for extremely severe Pott’s kyphotic deformity. Eur Spine J. 2009;18(10):1436-1441.
- Lu G, Wang B, Li Y, Li L, Zhang H, Cheng I. Posterior vertebral column resection and intraoperative manual traction to correct severe post-tubercular rigid spinal deformities incurred during childhood: minimum 2-year follow-up. Eur Spine J. 2015;24(3):586-593.
- Rajasekaran S, Vijay K, Shetty AP. Single-stage closing-opening wedge osteotomy of spine to correct severe post-tubercular kyphotic deformities of the spine: a 3-year follow-up of 17 patients. Eur Spine J. 2009;19(4):583-592.
- Zhang HQ, Li JS, Liu SH, et al. The use of posterior vertebral column resection in the management of severe posttuberculous kyphosis: a retrospective study and literature review. Arch Orthop Trauma Surg. 2013;133(9):1211-1218.
- Kim SS, Cho BC, Kim JH, et al. Complications of Posterior Vertebral Resection for Spinal Deformity. Asian Spine J. 2012;6(4):257.
- Jayaswal A, Upendra B, Ahmed A, Chowdhury B, Kumar A. Video-assisted Thoracoscopic Anterior Surgery for Tuberculous Spondylitis. Clin Orthop Relat Res. 2007;460:100-107.
- Kapoor S, Kapoor S, Agrawal M, Aggarwal P, Jain BK Jr. Thoracoscopic decompression in Pott’s spine and its long-term follow-up. Int Orthop. 2012;36(2):331-337.
- Yang H, Hou K, Zhang L, et al. Minimally invasive surgery through the interlaminar approach in the treatment of spinal tuberculosis: a retrospective study of 31 patients. J Clin Neurosci. 2016;32:9-13.
- Kalita J, Misra UK, Mandal SK, Srivastava M. Prognosis of conservatively treated patients with Pott’s paraplegia: logistic regression analysis. J Neurol Neurosurg Psychiatry. 2005;76(6):866-868.
- Mwachaka PM, Ranketi SS, Nchafatso OG, Kasyoka BM, Kiboi JG. Spinal tuberculosis among human immunodeficiency virus-negative patients in a Kenyan tertiary hospital: a 5-year synopsis. Spine J. 2011;11(4):265-269.
- Lerner PI. Nocardia species. In: Mandell GL, Douglas RGJ, Bennett JE, eds. Principles and Practice of Infectious Diseases. Vol 3rd. New York: Churchill Livingstone; 1990:1926-1932.
- Ito H, Tsuchiya J, Asami G. A new radical operation for Pott’s disease. J Bone Joint Surg Am. 1934;16(3):499-515.
- Smego RA Jr., Foglia G. Actinomycosis. Clin Infect Dis. 1998;26(6):1255-1261.
- Voisin L, Vittecoq O, Mejjad O, et al. Spinal abscess and spondylitis due to actinomycosis. Spine (Phila Pa 1976). 1998;23(4):487-490.
- Honda H, Bankowski MJ, Kajioka EHN, Chokrungvaranon N, Kim W, Gallacher ST. Thoracic vertebral actinomycosis: Actinomyces israelii and Fusobacterium nucleatum. J Clin Microbiol. 2008;46(6):2009-2014.
- Keenan JD, Metz CW Jr. Brucella spondylitis a brief review and case report. Clin Orthop Relat Res. 1972;82:87-91.
- Mikolich DJ, Bsyca JM. Brucella species. In: Mandell GL, Douglas RGJ, Bennett JE, (eds). Principles and Practice of Infectious Diseases. 3rd edn. Churchill Livingtone: New York, 1990, pp 1735-1742.
- Pritchard DJ. Granulomatous infections of bones and joints. Orthop Clin North Am. 1975;6(4):1029-1047.
- Lifeso RM, Harder E, McCorkell SJ. Spinal brucellosis. J bone Joint Surg Br. 1985;67(3):345-351.
- Bosilkovski M, Dimzova M, Grozdanovski K. Natural history of brucellosis in an endemic region in different time periods. Acta Clin Croat. 2009;48(1):41-46.
- Colmenero JD, Reguera JM, Martos F, et al. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine (Baltimore). 1996;75(4):195-211.
- Aygen B, Doğanay M, Sümerkan B, Yildiz O, Kayabaş Ü. Clinical manifestations, complications and treatment of brucellosis: a retrospective evaluation of 480 patients. Med Mal Infect. 2002;32(9):485-493. doi:10.1016/s0399-077x(02)00403-1.
- Hasanjani Roushan MR, Mohrez M, Smailnejad Gangi SM, Soleimani Amiri MJ, Hajiahmadi M. Epidemiological features and clinical manifestations in 469 adult patients with brucellosis in Babol, Northern Iran. Epidemiol Infect. 2004;132(6):1109-1114.
- Mantur BG, Amarnath SK, Shinde RS. Review of clinical and laboratory features of human Brucellosis. Indian J Med Microbiol. 2007;25(3):188-202.
- Bosilkovski M, Krteva L, Caparoska S, Dimzova M. Osteoarticular involvement in brucellosis: study of 196 cases in the Republic of Macedonia. Croat Med J. 2004;45(6):727-733.
- Geyik MF, Gur A, Nas K, et al. Musculoskeletal involvement of brucellosis in different age groups: a study of 195 cases. Swiss Med Wkly. 2002;132(7-8):98-105.
- Bosilkovski M, Kirova-Urosevic V, Cekovska Z, et al. Osteoarticular involvement in childhood brucellosis: experience with 133 cases in an endemic region. Pediatr Infect Dis J. 2013;32(8):815-819.
- Hashemi SH, Keramat F, Ranjbar M, Mamani M, Farzam A, Jamal-Omidi S. Osteoarticular complications of brucellosis in Hamedan, an endemic area in the west of Iran. Int J Infect Dis. 2007;11(6):496-500.
- Colmenero JD, Ruiz-Mesa J, Plata A, et al. Clinical findings, therapeutic approach, and outcome of brucellar vertebral osteomyelitis. Clin Infect Dis. 2008;46(3):426-433.
- Mousa AM, Bahar RH, Araj GF, et al. Neurological complications of brucella spondylitis. Acta Neurol Scand. 1990;81(1):16-23.
- Sharif HS, Aideyan OA, Clark DC, et al. Brucellar and tuberculous spondylitis: comparative imaging features. Radiology. 1989;171(2):419-425.
- Alp E, Doganay M. Current therapeutic strategy in spinal brucellosis. Int J Infect Dis. 2008;12(6):573-577.
- Pappas G, Seitaridis S, Akritidis N, Tsianos E. Treatment of brucella spondylitis: lessons from an impossible meta-analysis and initial report of efficacy of a fluoroquinolone-containing regimen. Int J Antimicrob Agents. 2004;24(5):502-507.
- Solera J, Martinez-Alfaro E, Espinosa A. Recognition and optimum treatment of brucellosis. Drugs. 1997;53(2):245-256.
- Chhem RK, Wang S, Jaovisidha S, et al. Imaging of fungal, viral, and parasitic musculoskeletal and spinal diseases. Radiol Clin North Am. 2001;39(2):357-378.
- Kim CW, Perry A, Currier B, Yaszemski M, Garfin SR. Fungal infections of the spine. Clin Orthop Relat Res. 2006;443:92-99.
- Ganesh D, Gottlieb J, Chan S, Martinez O, Eismont F. Fungal infections of the spine. Spine (Phila Pa 1976). 2015;40(12):E719-E728.
- O Guinn DJ, Serletis D, Kazemi N. Fungal osteomyelitis with vertebral re-ossification. Int J Surg Case Rep. 2016;19:1-3.
- Youmans JR, Winn HR. Youmans neurological surgery, 6th Ed. Saunders. 2011.
- Sharif HS. Role of MR imaging in the management of spinal infections. AJR Am J Roentgenol. 1992;158(6):1333-1345.
- Ottolenghi CE. Aspiration Biopsy of the Spine. Technique for the thoracic spine and results of twenty-eight biopsies in this region and over-all results of 1050 biopsies of other spinal segments. J Bone Joint Surg Am. 1969;51(8):1531-1544.
- Frazier DD, Campbell DR, Garvey TA, Wiesel S, Bohlman HH, Eismont FJ. Fungal Infections of the Spine. Report of eleven patients with long-term follow-up. J Bone Joint Surg Am. 2001;83(4):560-565.
- Smith CE, Beard RR, et al. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Heal Nations Heal. 1946;36(12):1394-1402.
- Smith CE. Epidemiology of acute coccidioidomycosis with erythema nodosum (“San Joaquin” or “Valley Fever”). Am J Public Heal Nations Heal. 1940;30(6):600-611.
- Johnson RH, Caldwell JW, Welch G, Einstein HE. The great coccidioidomycosis epidemic: clinical features. In: Coccidioidomycosis. Proceedings of the 5th International Conference. Bethesda, MD: National Foundation for Infectious Diseases; 1996:77-87.
- Stadalnik RC, Goldstein E, Hoeprich PD, dos Santos PA, Lee KK. Diagnostic value of gallium and bone scans in evaluation of extrapulmonary coccidioidal lesions. Am Rev Respir Dis. 1980;121(4):673-676.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23(2):367-381.
- Bariola JR, Perry P, Pappas PG, et al. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis. 2010;50(6):797-804.
- Moore RM, Green NE. Blastomycosis of bone. A report of six cases. J Bone Joint Surg. 1982;64(7):1097-1101.
- Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin Infect Dis. 1998;26(2):413-418.
- Johnson MD, Perfect JR. Fungal infections of the bones and joints. Curr Infect Dis Rep. 2007;3(5):450-460.
- Khazim RM, Debnath UK, Fares Y. Candida albicans osteomyelitis of the spine: progressive clinical and radiological features and surgical management in three cases. Eur Spine J. 2006;15(9):1404-1410.
- Vinas FC, King PK, Diaz FG. Spinal aspergillus osteomyelitis. Clin Infect Dis. 1999;28(6):1223-1229.
- Xing D, Ma JX, Ma XL, et al. A methodological, systematic review of evidence-based independent risk factors for surgical site infections after spinal surgery. Eur Spine J. 2013;22(3):605-615.
- Weinstein MA, McCabe JP, Cammisa FP Jr. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13(5):422-426.
- Veeravagu A, Patil CG, Lad SP, Boakye M. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976). 2009;34(17):1869-1872.
- Thalgott JS, Cotler HB, Sasso RC, LaRocca H, Gardner V. Postoperative infections in spinal implants classification and analysis--a multicenter study. Spine (Phila Pa 1976). 1991;16(8):981-984.
- Horan TC, Culver DH, Gaynes RP, Jarvis WR, Edwards JR, Reid CR. Nosocomial infections in surgical patients in the United States, January 1986-June 1992. National Nosocomial Infections Surveillance (NNIS) System. Infect Control Hosp Epidemiol. 1993;14(2):73-80.
- Pull ter G, Mohamed AS, Skolasky RL, van Laarhoven CJHM, Cohen DB. The presentation, incidence, etiology, and treatment of surgical site infections after spinal surgery. Spine (Phila Pa 1976). 2010;35(13):1323-1328.
- Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976). 2005;30(12):1460-1465.
- Smith JS, Shaffrey CI, Sansur CA, et al. Rates of infection after spine surgery based on 108,419 procedures:a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976). 2011;36(7):556-563.
- Silber JS, Anderson DG, Vaccaro AR, Anderson PA, McCormick P. Management of postprocedural discitis. Spine J. 2002;2(4):279-287.
- Bassewitz HL, Fischgrund JS, Herkowitz HN. Postoperative spine infections. Semin Spine Surgery. 2000; 12: 203-11.
- Chaudhary SB, Vives MJ, Basra SK, Reiter MF. Postoperative spinal wound infections and postprocedural diskitis. J Spinal Cord Med. 2007;30(5):441-451.
- Pawar AY, Biswas SK. Postoperative Spine Infections. Asian Spine J. 2016;10(1):176-183.
- Dubousset J, Shufflebarger H, Wenger D. Late “infection” with CD instrumentation. Orthop Trans. 1994;18(3):121-126.
- Sponseller PD, LaPorte DM, Hungerford MW, Eck K, Bridwell KH, Lenke LG. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine (Phila Pa 1976). 2000;25(19):2461-2466.
- Gerometta A, Bittan F, Rodriguez Olaverri JC. Postoperative spondilodiscitis. Int Orthop. 2012;36(2):433-438.
- Richards BS, Herring JA, Johnston CE, Birch JG, Roach JW. Treatment of adolescent idiopathic scoliosis using Texas Scottish Rite Hospital instrumentation. Spine (Phila Pa 1976). 1994;19:1598-1605.
- Singh K, Heller JG. Postoperative Spinal Infections. Contemp Spine Surg. 2005;6(9):61-68.
- Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surgery Am. 2011;93(17):1627-1633.
- Clark NM, Hershberger E, Zervosc MJ, Lynch JP 3rd. Antimicrobial resistance among gram-positive organisms in the intensive care unit. Curr Opin Crit Care. 2003;9(5):403-412.
- Browne JA, Cook C, Pietrobon R, Bethel MA, Richardson WJ. Diabetes and early postoperative outcomes following lumbar fusion. Spine (Phila Pa 1976). 2007;32(20):2214-2219.
- Capen DA, Calderone RR, Green A. Perioperative risk factors for wound infections after lower back fusions. Orthop Clin North Am. 1996;27(1):83-86.
- Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord. 1998;11(2):124-128.
- Abdul-Jabbar A, Takemoto S, Weber MH, et al. Surgical site infection in spinal surgery: description of surgical and patient-based risk factors for postoperative infection using administrative claims data. Spine (Phila Pa 1976). 2012;37(15):1340-1345.
- Zusman N, Munch JL, Ching A, Hart R, Yoo J. Preoperative epidural spinal injections increase the risk of surgical wound complications but do not affect overall complication risk or patient-perceived outcomes. J Neurosurg Spine. 2015;23(5):652-655.
- Yang S, Werner BC, Cancienne JM, et al. Preoperative epidural injections are associated with increased risk of infection after single-level lumbar decompression. Spine J. 2016;16(2):191-196.
- Huang RC, Shapiro GS, Lim M, Sandhu HS, Lutz GE, Herzog RJ. Cervical epidural abscess after epidural steroid injection. Spine (Phila Pa 1976). 2004;29(1):E7-9.
- Horwitz NH, Curtin JA. Prophylactic antibiotics and wound infections following laminectomy for lumber disc herniation. J Neurosurg. 1975;43(6):727-731.
- Carreon LY, Puno RM, Lenke LG, et al. Non-neurologic complications following surgery for adolescent idiopathic scoliosis. J Bone Jt Surgery-American Vol. 2007;89(11):2427-2432. doi:10.2106/00004623-200711000-00013.
- Beiner JM, Grauer J, Kwon BK, Vaccaro AR. Postoperative wound infections of the spine. Neurosurg Focus. 2003;15(3):E14.
- Kulkarni GS ed. Textbook of Orthopaedics and Trauma, Vol 1. New Delhi: Jaypee Brothers Medical Pub; 2008.
- Simopoulos TT, Kraemer JJ, Glazer P, Bajwa ZH. Vertebral osteomyelitis: a potentially catastrophic outcome after lumbar epidural steroid injection. Pain Physician. 2008;11(5):693-697.
- Cancienne JM, Werner BC, Puvanesarajah V, et al. Does the timing of preoperative epidural steroid injection affect infection risk after ACDF or posterior cervical fusion? Spine J. 2016;16(10):S133-S134.
- Pull ter G, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976). 2009;34(13):1422-1428.
- Watters WC 3rd, Baisden J, Bono CM, et al. Antibiotic prophylaxis in spine surgery: an evidence-based clinical guideline for the use of prophylactic antibiotics in spine surgery. Spine J. 2009;9(2):142-146.
- Keller RB, Pappas AM. Infection after spinal fusion using internal fixation instrumentation. Orthop Clin North Am. 1972;3(1):99-111.
- Block C, Robenshtok E, Simhon A, Shapiro M. Evaluation of chlorhexidine and povidone iodine activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis using a surface test. J Hosp Infect. 2000;46(2):147-152.
- Goldenheim PD. In vitro efficacy of povidone-iodine solution and cream against methicillin-resistant Staphylococcus aureus. Postgrad Med J. 1993;69 Suppl 3:S62-65.
- Haley CE, Marling-Cason M, Smith JW, Luby JP, Mackowiak PA. Bactericidal activity of antiseptics against methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985;21(6):991-992.
- Hill RLR, Casewell MW. The in-vitro activity of povidone-iodinecream against Staphylococcus aureus and its bioavailability in nasal secretions. J Hosp Infect. 2000;45(3):198-205.
- McLure AR, Gordon J. In-vitro evaluation of povidone-iodine and chlorhexidine against methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1992;21(4):291-299.
- Reimer K, Wichelhaus TA, Schafer V, et al. Antimicrobial effectiveness of povidone-iodine and consequences for new application areas. Dermatology. 2002;204(1):114-120.
- Cheng MT, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976). 2005;30(15):1689-1693.
- Lee GI, Bak KH, Chun HJ, Choi KS. Effect of using local intrawound vancomycin powder in addition to intravenous antibiotics in posterior lumbar surgery: midterm result in a single-center study. Korean J Spine. 2016;13(2):47-52.
- Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976). 2011;36(24):2084-2088.
- Molinari RW, Khera OA, Molinari WJ 3rd. Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J. 2012;21 Suppl 4:S476-482.
- O’Neill KR, Smith JG, Abtahi AM, et al. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J. 2011;11(7):641-646.
- Jo JE, Miller AO, Cohn MR, Nemani VM, Schneider R, Lebl DR. Evaluating the diagnostic yield of computed tomography-guided aspirations in suspected post-operative spine infections. HSS J. 2016;12(2):119-124.
- Basu S, Ghosh J, Malik F, Tikoo A. Postoperative discitis following single-level lumbar discectomy: our experience of 17 cases. Indian J Orthop. 2012;46(4):427-433.
- Glassman SD, Dimar JR, Puno RM, Johnson JR. Salvage of instrumented lumbar fusions complicated by surgical wound infection. Spine (Phila Pa 1976). 1996;21(18):2163-2169.
- Mok JM, Guillaume TJ, Talu U, et al. Clinical outcome of deep wound infection after instrumented posterior spinal fusion: a matched cohort analysis. Spine (Phila Pa 1976). 2009;34(6):578-583.

