Nicolas Dea and Charles Fisher
INTRODUCTION
Primary bone neoplasms of the spine are rare; they represent four to thirteen percent of all primary bone tumors. Incidence of primary bone neoplasms is estimated at 2.5–8.5/100,000 people a year.1 Due to the rarity of primary spine tumors, the experience of most surgeons with these tumors is limited, thus leading to inconsistencies in approach to diagnosis and treatment. High index of suspicion is crucial because a curable tumor can be rendered incurable if managed inappropriately. Therefore, clinical expertise is concentrated in selected referral centers with appropriate multidisciplinary treatment teams.
In the adult population, malignant tumors are more prevalent as opposed to in the pediatric population, where benign tumors are more common. The most common primary malignant tumors arising from the spine are chordomas, osteosarcomas, chondrosarcomas and Ewing’s sarcomas. In order of frequency, the most prevalent benign tumors of the spine are osteoblastomas, aneurysmal bone cysts and osteoid osteomas. Due to its highest number of vertebrae, the thoracic spine is the most common localization for primary tumors as a whole. However, some tumors have predilections for specific regions of the spinal column. Arising from remnants of the embryonic notochord, chordomas are most commonly found at the extremities of the spine: namely, the clival and sacral regions. Osteosarcomas and giant cell tumors are also commonly found in the sacrum. Lastly, osteosarcomas are most common in patients with Paget’s disease.
DIAGNOSIS
Mean age at presentation is forty-two years; fifty-two percent are male and forty-eight percent are female. The most common presenting symptom is pain, affecting 76% of the benign tumors and 95% of the malignant tumors. Pain can be oncologic, mechanical or neurologic in nature and it is important to differentiate between the three.
Oncologic pain is related to the tumor itself: it can be secondary to the release of chemical mediators, increased pressure within the bone, micro-fractures, stretching of the periosteum, or reactive muscle spasms. Oncologic pain is unchanged with position and often worsens at night secondary to venous congestion and cortisol nadir. Mechanical pain is secondary to acquired instability caused by tumor involvement of the bony complex and/or destruction of the disco-ligamentous complex. The pain increases with movement and when the patient is in the upright or sitting position. Lastly, neurologic pain is caused by compression of the neural elements and will often follow a known dermatome when a root is compressed and can be dysesthetic in nature. Thoracic radicular pain may be confounded with chest pain leading to pulmonary or cardiac investigations. Neurological deficits occur when the neural elements are compressed by either the tumor or by retropulsed bone fragments from a pathological fracture. The degree of neurologic compromise can vary significantly from radicular numbness to complete quadriplegia. Bowel and bladder disturbance alone can occur in sacral tumors. Painful deformity can be the present symptom of an osteoid osteoma in an adolescent.
The main differential diagnosis of a primary bone tumor is metastatic disease, which is significantly more common. Solitary bone metastasis of unknown origin may pose a diagnostic challenge, and should be considered a potential primary tumor until proven otherwise. Other differential diagnoses include infections, metabolic disorders, and degenerative processes. Meticulous history and physical examination, supplemented by appropriate diagnostic tests, give the treating physician a working diagnosis requiring histological confirmation. In a young patient, a benign origin should be suspected, especially when the tumor arises from the posterior elements. Exceptions to the forgoing are Ewing sarcomas and osteosarcomas, both having a predilection for the pediatric population. Most vertebral body tumors in an adult will be malignant, likely metastatic lesions or multiple myelomas, but also rarer primary tumors like chordomas and chondrosarcomas. Hemangioma is one exception—a benign tumor often found in the anterior column in the adult population.
Radiology is central in the management of patients with suspected primary bone tumors. The following tests are used to identify various elements and aspects of a tumor. Thirty-six-inch standing plain X-rays give valuable insight to the presence of dynamic instability and overall sagittal balance, both of which are important when planning reconstruction of the spine. High resolution multiplanar computed tomography (CT) is the most accurate imaging modality to assess the bony quality of a tumor and to identify pathological fractures. Whole spine magnetic resonance imaging (MRI) is best to evaluate the epidural component of a tumor, as well as the degree of neural element compression and soft tissue extent. Positron emission tomography (PET) and bone scan may prove very useful in the assessment of a patient with a suspected primary spine tumor. Both tests are useful to monitor metabolic activity of a tumor and rule out a metastatic disease. The vascularity of a tumor, as well as localization and involvement of important vascular branches, is better assessed by catheter angiography. Embolization of a vascular tumor is also of great help and can be performed at the same time.
APPROACH
There are a variety of aspects involved in obtaining a diagnosis. Initial management of a suspected primary tumor consists of local and systemic staging culminating in a well-planned CT-guided trocar biopsy. Open biopsy is correlated with an increased risk of local recurrence and should therefore be avoided.2 Poorly planned biopsies or those done outside of the center where definitive care will occur are independent risk factors for tumor recurrence.3 Biopsy tract may often need to be excised. As such, the oncologic spinal surgeon should be involved in biopsy planning when a primary spine tumor is suspected. Results from the local and systemic staging, combined with histological diagnosis made by an experienced musculoskeletal pathologist, allow the tumor to be classified according to the Enneking classification.
The Enneking classification was originally described for tumors of the appendicular skeleton. However, this classification system is still reliable and valid when applied to a primary spine tumor.4-5 Enneking classification is based on the interrelationship of 3 factors: a grade of biological aggressiveness (G), its local extent (T), and the presence or absence of metastasis (M) (Table 1-1).
| Stage | Grade | Local Extension | Metastases |
|---|---|---|---|
| Benign | |||
| 1 | G0 | Intracompartmental (T0) | No (M0) |
| 2 | G0 | Intracompartmental (T0) | No (M0) |
| 3 | G0 | Intra- or extracompartmental (T1/T2) | No (M0) |
| Malignant | |||
| 1A | Low (G1) | Intracompartmental (T1) | No (M0) |
| 1B | Low (G1) | Extracompartmental (T2) | No (M0) |
| 2A | High (G2) | Intracompartmental (T1) | No (M0) |
| 2B | High (G2) | Extracompartmental (T2) | No (M0) |
| 3 | Any | Any | Yes (M1) |
Histological, radiological and clinical characteristics correlate to identify a benign (G0), low-grade malignant (G1), or a high-grade malignant tumor (G2). Benign lesions are further subcategorized as latent (S1), active (S2) or aggressive (S3), based on the tumor-host margin. Local extent is described as intracapsular (T0), intracompartmental (T1) or extracompartmental (T2). A combination of these three factors determines the Enneking stage, for which a specific surgical resection margin +/- adjuvant treatment is recommended (Table 1-2).5
| Enneking Stages | Margin for Control |
| 1 | No management unless for decompression or stabilization |
| 2 | Intralesional excision ± local adjuvants |
| 3 | Marginal en bloc excision |
| IA | Wide en bloc excision |
| IB | Wide en bloc excision |
| IIA | Wide en bloc excision + effective adjuvants |
| IIB | Wide en bloc excision + effective adjuvants |
| IIIA | Palliative |
| IIIB | Palliative |
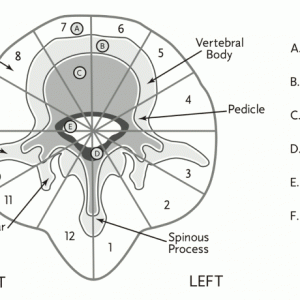
Weinstein-Boriani-Biagini (WBB) classification is used for surgical staging (Fig. 1-1). This classification addresses the unique complexity of tumors originating from the spinal column.6 WBB assists in surgical planning by establishing feasibility criteria and strategies for achieving appropriate oncological resection while preserving neurological function based on the location of the tumor within the vertebrae.
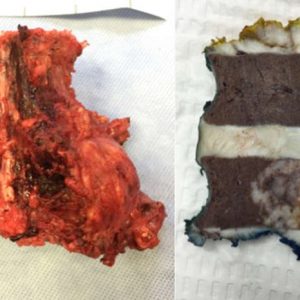
In order to study primary spine tumors, one must know the fundamental standardized terminology. En bloc resection refers to an attempt at resecting the tumor in one piece, as opposed to curettage or piecemeal resection which means a deliberate intralesional resection. However, en bloc resection on its own without a pathological margin description has little value.
Margins can be intralesional, marginal, or wide. Intralesional means the plane of dissection has transgressed into the lesion. Marginal is when the dissection is carried within the reactive zone or pseudocapsule surrounding the tumor. Wide margin is when the plane of dissection is within normal tissue, beyond the reactive zone.
Multidisciplinary teams are crucial in the diagnosis and handling of these tumors. Primary spinal tumors should be managed in experienced quaternary care centers with appropriate multidisciplinary teams. Each case should be discussed in rounds attended by the surgeon, radiation oncologist, medical oncologist, pathologist and radiologist. Often, surgical execution will require other surgical subspecialties to assist the oncologic spine surgeon.
NON-SURGICAL TREATMENT
Non-surgical treatment options for primary spine tumors are many. As a general rule, when neoadjuvant therapy is indicated, it is recommended to restage after the therapy, and then determine the surgical course of treatment. Considerable medical advances are changing treatment paradigms for different primary spinal tumors.
Multinucleated giant cells found in giant cell tumors (GCT) have been found to express high level of an essential mediator in bone resorption called RANK-ligand. Denosumab is a monoclonal antibody that inhibits RANKL and is often used to treat osteoporosis. The use of Denosumab to treat GCT has been correlated with good disease control and calcification and shrinkage of the tumor, which allows for an easier and potentially less morbid surgical resection.7 This new data supports the use of this drug as a neoadjuvant treatment for GCT, as well as for inoperable GCT. Optimal duration of treatment and long-term side effects of treatment are yet to be determined. Other drugs like alpha-2B interferon and bisphosphonate are being studied for the treatment of GCT but are currently not recommended as routine first-line treatments.8
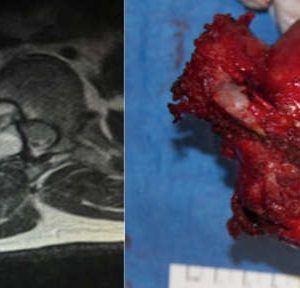
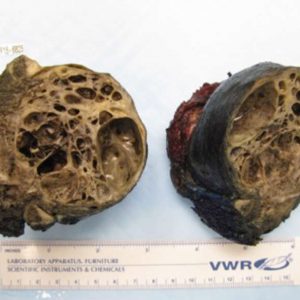
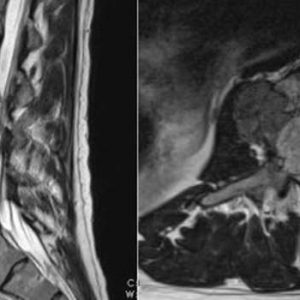
Since Aneurismal bone cyst (ABC) and GCT may share common osteolytic features, Denosumab is also under study in the management of ABC, but due to very low evidence at the moment, cannot be recommended as first line treatment for this type of tumor (Fig. 1-2 and Fig. 1-3). Doxycycline is an antibiotic with anti-tumoral properties. When injected intra-lesionally, it has been shown to be associated with re-ossification and radiological improvement in ABC. It is, however, reserved for inoperable and recurrent lesions and is not recommended as first-line treatment.
Percutaneous/interventional radiology is another form of non-surgical treatment. Preoperative angiography and embolization are instrumental to many primary spine tumor cases to localize important arterial feeders (e.g., artery of Adamkiewicz) and to reduce blood flow to a vascular tumor. Occlusion of a peri-tumoral segmental artery may also assist in surgical resection by facilitating tumoral dissection. Moreover, interventional radiology is often involved to perform CT-guided trocar biopsies. Percutaneous treatment can also be a definitive or an adjuvant treatment option in certain circumstances.
Aneurysmal bone cyst is conventionally treated with pre-operative embolization followed by gross total resection. Selective arterial embolization as a stand-alone treatment has recently emerged as a new treatment possibility. Multiple embolizations are often required, which raises the issue of radiation exposure, especially in the pediatric population. Percutaneous injection of bone marrow aspirate and demineralized bone matrix is also being studied and could be a promising treatment alternative, especially in recurrent and unresectable cases.8
Percutaneous thermal ablation of spinal osteoid osteoma (OO) is a minimally invasive treatment option correlated to very low recurrence rates. Special precautions, such as cooling epidural irrigation, are indicated in the absence of intact cortex and when the tumor is in close proximity to neural structures.
Radiation therapy (RT) is a very important non-surgical treatment. Neoadjuvant and/or adjuvant radiation therapy is often considered in chordoma and chondrosarcoma of the spine to increase local control. Specifically, high-dose conformal radiation therapy should be used when surgical margins are of concern, in all recurrent tumors and when surgery is not feasible. It may also be used in Enneking appropriate (EA) resection to reduce local recurrence (LR).
High radiation doses of up to 70 Gy are required to overcome the radioresistance of these tumors. Due to its Bragg-peak effect, proton beam RT allows high doses of radiation to be delivered to the tumor while preserving surrounding critical structures and has been extensively studied for chordomas of the spine. New RT modalities, such as photon-based intensity-modulated RT, are becoming available with advances in image guidance and radiation delivery and planning strategies. Just like proton-based treatment, these advances permit the required conformational high dose of radiation to be delivered to the tumor.10
Carbon ion therapy is a heavy charged-particle-based treatment, which seems to have a biological advantage compared with other forms of RT techniques. Promising early results are becoming available and show that carbon-ion therapy may even become a viable alternative to surgical treatment.
With many new molecular markers being discovered, targeted therapy is changing the way cancer is treated. Molecular signature of primary spine tumors is also extensively studied.11 Positive expression of Aurora kinase A and B was found to be an independent factor of poor survival in chondrosarcomas. Loss of RUNX3, a tumor suppressor gene, has also been associated with a more aggressive behavior in this tumor type. A single nucleotide polymorphism (SNP) in the T-gene, central in chordomas pathogenesis, is strongly linked to chordoma formation. Patients with the A variant of the SNP seem to have improved survival compared to those lacking the variant.12
SURGICAL PRINCIPLE
Despite remarkable advances in many other fields, surgery still plays a key role in the management of most primary spinal tumors. The inherent anatomy of the spine, combined with the need to maintain stability and neurological function, give rise to very significant surgical challenges, even in the most experienced hands. Respecting Enneking principles (Enneking Appropriate resection – EA) is correlated to lower recurrence rates and increased median survival for most primary spine tumors. It should therefore be the goal of treatment when facing such tumors to respect these principles.13,14
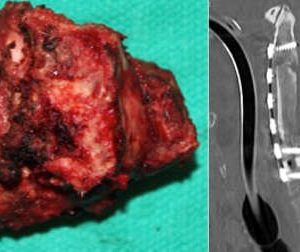
Next, the feasibility of en bloc resection will be assessed (Fig. 1-4 and Fig. 1-5). Each spine tumor has unique characteristics and each case should be approached individually. Two conditions are necessary to achieve a resection adhering to Enneking principles. First, the spinal column should be considered as a ring through which the neural elements pass. To preserve neurologic function, a tumor-free window must be created in that ring, through which the spinal cord can be spared as the tumor is being delivered. Second, access is needed to the nerve root at the dural margin via a clear plane of dissection between the tumor and the dura when amputation of a root is necessary. In the cervical spine, one vertebral artery must be preserved and therefore is a third necessary condition.
Safety and efficacy of surgery for primary tumors is of the upmost importance. Respecting oncologic principles for primary tumors of the spine is correlated with high adverse event (AE) rates of up to 100% for sacral tumors, and 74% for mobile spine tumors. Complication-related death of up to 27% and 7.7% has been reported for sacral and mobile spine tumors, respectively.16
Factors associated with increased incidence of AE are: length of surgery, previous surgery, type of resection (en bloc versus intralesional), previous irradiation, nutritional status, and patient comorbidities. Due to inherent particularities (proximity to the rectum, amount of dead space created, compromised continence), sacral tumor morbidity profile is dominated by wound infection. The average incidence of postoperative wound infection is 36%.
To minimize AE, the following are tools available to the spine surgeon:
- thorough, exhaustive surgical and anesthetic planning and multidisciplinary support;
- plastic surgery assistance and proper flap coverage when indicated;
- Vancomycin powder and vacuum assisted closure (VAC);
- surgical navigation;
- good reconstruction techniques;
- preoperative angiography; and
- embolization.
The following surgical results come from recent multicenter AO Spine Knowledge Forum Tumor studies. Mean post-operative overall survival (OS) for sacral chordoma17 is 6 years; mobile spine chordoma18 is 7 years; chondrosarcoma19 is 8.4 years; osteosarcoma20 is 6.7 years. Mean progression free survival (PFS) for sacral chordoma is 4 years; mobile spine chordoma is 5.3 years; chondrosarcoma: 35% at 10 years (mean PFS not reached during analysis); osteosarcoma: 30% at 3.5 years (mean PFS not reached during analysis).
There are also special anatomical considerations. Tumors of the cervical spine (Fig. 1-6) pose significant challenges due to presence of the vertebral artery, peculiar bony architecture, and the functional importance of cervical nerve roots. There are higher morbidity and mortality rates, as well as higher Enneking inappropriate (EI) resections tumors in this location.
Unilateral vertebral artery occlusion is usually well tolerated in patients with normal vascular anatomy. Endovascular balloon occlusion test, cerebral blood flow studies, and intra-operative temporary occlusion with neuromonitoring are strategies to assess if occlusion can be safely performed.
Complex soft tissue and spino-pelvic reconstructions are often necessary in the sacral region. Soft tissue reconstruction (rotational gluteal or transpelvic vertical rectus abdominis myocutaneous (VRAM) flaps) should be used to obliterate the dead space created after total sacrectomies. Spino-pelvic reconstruction should be undertaken after total sacrectomy when there is no preservation of ilio-lumbar ligaments.21 Preservation of bowel and bladder function occurs in the majority of patients following unilateral sacral roots resection or if at least one S3 root is preserved in the case of bilateral sacral resection.22
Tumor recurrence is associated with lower Health Related Quality of Life (HRQOL) scores more so than the morbidity of the surgical intervention itself. When long follow-up is available, HRQOL returns to normative values. Therefore, primary tumors of the spine should be treated with curative intent whenever possible, even at the expense of initial surgical morbidity.16
PEARLS AND PITFALLS
- Primary spinal tumor surgery, due to its very challenging and risky nature, should be performed in an experienced center with multidisciplinary support.
- Respecting evidence-based oncological principles results in lower rates of local recurrence and improved survival for most primary spine tumor patients.
- It is crucial to recognize a potential primary spine tumor and to appropriately obtain an accurate histological diagnosis. Not doing so could render a curable tumor incurable.
- The first operation is associated with the best chance of achieving cure with the least morbidity.
- Advances in many fields of oncology position primary spine tumors at the forefront of multidisciplinary care. Individualized treatment strategies originating from these different domains and incorporating patient preferences should be made for each case.
REFERENCES
- Dreghorn CR, Newman RJ, Hardy GJ, Dickson RA. Primary tumors of the axial skeleton. Experience of the Leeds Regional Bone Tumor Registry. Spine. 1990;15(2):137-140.
- Yamazaki T, McLoughlin GS, Patel S, Rhines LD, Fourney DR. Feasibility and safety of en bloc resection for primary spine tumors: a systematic review by the Spine Oncology Study Group. Spine. 2009;34(22 Suppl):S31-S38.
- Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88(9):2122-2134.
- Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;(204):9-24.
- Chan P, Boriani S, Fourney DR, et al. An assessment of the reliability of the Enneking and Weinstein-Boriani-Biagini classifications for staging of primary spinal tumors by the Spine Oncology Study Group. Spine. 2009;34(4):384-391.
- Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine: terminology and surgical staging. Spine. 1997;22(9):1036-1044.
- Goldschlager T, Dea N, Boyd M, et al. Giant cell tumors of the spine: has denosumab changed the treatment paradigm. J Neurosurg Spine. 2015;22(5):526-533.
- Charest-Morin R, Boriani S, Fisher CG, et al. Benign tumors of the spine: has new chemotherapy and interventional radiology changed the treatment paradigm? Spine. 2016;41 Suppl 20:S178-S185.
- Sciubba DM, Okuno SH, Dekutoski MB, Gokaslan ZL. Ewing and osteogenic sarcoma: evidence for multidisciplinary management. Spine. 2009;34(22 Suppl):S58-S68.
- Pennicooke B, Laufer I, Sahgal A, et al. Safety and local control of radiation therapy for chordoma of the spine and sacrum: a systematic review. Spine. 2016;41 Suppl 20:S186-S192.
- Goodwin CR, Abu-Bonsrah N, Bilsky MH, et al. Clinical decision making: integrating advances in the molecular understanding of spine tumors. Spine. 2016;41 Suppl 20:S171-S177.
- Bettegowda C, Yip S, Lo SL, et al. Spinal column chordoma: prognostic significance of clinical variables and T (brachyury) gene SNP rs2305089 for local recurrence and overall survival. Neuro Oncol. 2017;19(3): 405-413.
- Fisher CG, Keynan O, Boyd MC, Dvorak MF. The surgical management of primary tumors of the spine: initial results of an ongoing prospective cohort study. Spine. 2005;30(16):1899-1908.
- Fisher CG, Saravanja DD, Dvorak MF, et al. Surgical management of primary bone tumors of the spine: validation of an approach to enhance cure and reduce local recurrence. Spine. 2011;36(10):830-836.
- Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31(4):493-503.
- Dea N, Charest-Morin R, Sciubba DM, et al. Optimizing the adverse event and HRQOL profiles in the management of primary spine tumors. Spine. 2016;41 Suppl 20:S212-S217.
- Varga PP, Szövérfi Z, Fisher CG, et al. Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Eur Spine J. 2015;24(5):1092-1101.
- Gokaslan ZL, Zadnik PL, Sciubba DM, et al. Mobile spine chordoma: results of 166 patients from the AOSpine Knowledge Forum Tumor database. J Neurosurg Spine. 2016;24(4):644-651.
- Fisher CG, Versteeg AL, Dea N, et al. Surgical management of spinal chondrosarcomas. Spine. 2016;41(8):678-685.
- Dekutoski MB, Clarke MJ, Rose P, et al. Osteosarcoma of the spine: prognostic variables for local recurrence and overall survival, a multicenter ambispective study. J Neurosurg Spine. 2016;25(1):59-68.
- Reynolds JJ, Khundkar R, Boriani S, et al. Soft tissue and bone defect management in total sacrectomy for primary sacral tumors. Spine. 2016;41 Suppl 20:S199-S204.
- Todd LT Jr, Yaszemski MJ, Currier BL, Fuchs B, Kim CW, Sim FH. Bowel and bladder function after major sacral resection. Clin Orthop Relat Res. 2002;(397):36-39.