Nozomu Inoue, Alejandro A. Espinoza Orías, Kazuyuki Segami, and Howard S. An
INTRODUCTION
Facet joints, also known as zygapophysial or apophyseal joints, are true synovial joints that can undergo degenerative changes in a similar fashion to other synovial joints. The unique morphology of the facet joint is linked to its biomechanical function. Failure of the facet joint’s biomechanical function leads to osteoarthritic changes and is implicated in other spinal disorders, such as degenerative spondylolisthesis. This chapter will summarize the functional anatomy and biomechanics of the lumbar facet joint and biomechanical pathogenesis of degenerative spondylolisthesis caused by dysfunction of the facet joint.
FUNCTIONAL ANATOMY OF THE LUMBAR FACET JOINT
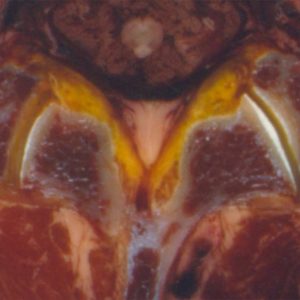
The facet joints comprise the inferior and superior articular processes, which are bony protuberances that arise vertically from the junction of pedicles and laminae behind the transverse processes (Fig. 10-1). The articular processes are completely incorporated into the laminae in the lumbar region, so that the loads passing from superior to inferior articular facets diffuse into the lamina.1 Distinctive trabecular orientation is recognized running obliquely from the superior process downward to the inferior endplate and from the inferior process upward to the superior endplate in the sagittal plane.2,3 Furthermore, trabecular orientation mainly perpendicular to the articular surface in the transverse plane has been recognized in the superior process.4,5
Articulating cartilage covering the superior and inferior articular processes primarily faces posteromedial and anterolateral directions, respectively. However, it was reported that the articulating cartilage does not extend all the way to the tips of the processes,6 which sometimes makes the definition of the articular cartilage contours difficult. The articulating cartilage in the processes appears to be suitable to transmit the forces in the transverse plane parallel to the endplates, rather than transmitting the vertical force applied to the posterior element of the spinal column considering its orientation and distribution. However, Hadley reported that the articular cartilage extended beyond the limits of bony contact, which enlarges the joint space extending around to the posterior surface of the articular process.7
The anatomical definition of the term ‘‘facet’’ is a smooth flat circumscribed anatomical surface. Since articular surfaces on the superior and inferior articular processes are generally described as concave and convex, respectively, and are effectively not flat, the term “facet joint” may not represent well the geometrical characteristics of the zygapophysial joint.8 The facet joint in the transverse plane approximates a “C” or “J” shape. While it has been reported that approximately 80% of the facet joints are curved and 20% are flat in the upper lumbar spine, these numbers are reversed in the lower lumbar spine.9 Hadley showed the existence of concave articular surfaces in the inferior facet and convex articular surfaces in the superior facet in the sagittal plane, which are opposite curvatures in the transverse plane and conflict with the generally considered geometry of the facet joint surfaces.7 A surface inversely curved in two perpendicular directions, i.e., in one concave and in the other convex, is described as a “saddle” shape, which is described mathematically as a hyperbolic paraboloid. In fact, Steindler classified the lumbar facet joint as a saddle joint in his textbook.3
As is the case with other major synovial joints, the facet joint has a capsule. The capsule consists of an outer layer made of densely packed parallel bundles of collagen fibers and an inner layer of irregularly oriented wavy elastic fibers.10 The parallel bundles have been described as firm “transverse strengthening ligaments” by Putz.4 A recent study on the detailed lumbar capsular structures showed three bands of fibers in the outer layer of the joint capsule; superior curved fibers, middle horizontal running fibers and inferior curved fibers.11 The superior curved fibers form a distinct “dome-like band” that crosses the superior and superior-posterior parts of the joint. The middle horizontal fibers generally run horizontally but may also slant slightly downwards from medial to lateral. The inferior curved fibers form a distinct “hammock-like band” that crosses the inferior and posterior-inferior part of the facet joint.11
The capsule is attached not to the margins of the joints but reflected around to the outer surfaces of the articular processes since articular cartilage extends beyond the posterior surface of the articular process.7 The capsule and joint space extend a variable distance from the margins along the superior or inferior articular process.12 Attachment of the capsule at a certain distance from the margin of the joint surface causes a “wrap-around” effect of the capsule, which can create compressive forces and stresses within the capsule, and thus fibrocartilage can be formed in the capsule.13
A study on the thickness of the lumbar facet capsule reported a regional variation of the thickness; 2.0 mm thick in the posterior region, 2.4 mm thick in superior and inferior regions, whereas as much as 3.2 mm thick in the anterior region.14 The regional variation of the capsule thickness was also noted in the study10 above with the inner layer in the inferior part thicker than that in the superior and middle parts of the joint. However, this finding was only qualitative.
Proper knowledge of both the anatomical geometry of the facet joint surface and capsular structures are important inputs for biomechanical studies that seek to elucidate normal function and dysfunction causing osteoarthritis of the facet joint and spinal disorders.
BIOMECHANICAL FUNCTION OF THE FACET JOINTS
Axial Load Transmission through the Facet Joint
The facet joints and the intervertebral discs provide physiological spinal motions and protect the spine by preventing activities that can be injurious by functioning together. A structure consisting of the facet joints and the intervertebral discs has been called the spinal motion segment, the three-joint complex, or the articular triad.15,16 While the intervertebral disc has usually been considered to transmit mostly an axial (vertical) compressive load placed on the back, the facet joints have been traditionally considered to primarily function in guiding and stabilizing the motion segment.1,17-19 The relative magnitude of the axial compressive force passing through the intervertebral disc and facet joints has been studied by several investigators, demonstrating that there is some type of load transmission through the facet joints. Adams and Hutton showed 16% of the whole spine load is transmitted through the facet joints when the lumbar spine is in slight extension of 2°, as in an erect standing position, and after the intervertebral disc height has been reduced by a period of axial compressive loading, whereas no load is transmitted via the facet joint in slight flexion, as in an erect sitting posture.6
The same research group measured contact pressure between facet joint surfaces with pressure-sensitive paper under different postures and reduced disc height by nucleotomy and showed increased peak pressure with disc height loss and increasing extension.6,20 Dunlop et al. reported the contact pressure in the human cadaveric lumbar facet joint with a 1000N compressive load and a shear load of 200N to 400N and reported contact pressures of 6.1 MPa in the central-medial and central-inferior regions of the articular surface near its periphery for 6° of extension.20
Axial compressive load transmission through direct contact between the tips of articular processes and the neural arch (i.e., the lamina or the pars interarticularis) is important when the lumbar spine is extended and/or the height of the intervertebral disc is reduced because the distance between the tips of the articular processes and the neural arch becomes narrower in such circumstances. A histological study by Hadley demonstrated articulation between the tip of the superior articular process and the pedicle of the adjacent superior vertebra or between the tip of the inferior articular process and the lamina or the pars interarticularis of the adjacent inferior vertebra caused by telescoping or imbrication of the facet articulations in the lumbar segment with intervertebral disc height loss.7 In these facet joints, the original facet articular cartilage layers do not register exactly opposite each other, and the development of a fibrocartilage bumper due to intermittent pressure was noted at the tips of the articular processes.7 Yang and King estimated that 3-25% of axial compressive load is carried via the normal facets by an indirect method using cadaveric lumbar specimens.21 The authors claimed that the mechanism of load transmission is due to the bottoming-out of the tip of the inferior facet on the pars interarticularis of the vertebra below. Dunlop et al. also stated that substantial load might be transmitted from the tips of the facets directly to the lamina below, or to the pars interarticularis above.20 In the experiment by Dunlop et al., extra-articular impingement was only seen in maximum extension at full disc height, but when the disc had been made narrower it was found in all postures. The load transmission mechanism across the facet joint through bony contact was proven by direct measurement of facet tip contact pressure using a pressure transducer implemented at the tip of a 13-gauge steel tube, which was placed at the tip of the inferior articular process through the bone part of the process.22
Information on the axial compression load transmission through the capsular ligaments is limited in the literature. Ivicsics et al. measured load transmission through the facet joint with and without capsular ligaments under 700N axial compression in extension-flexion (neutral position ±5° with 0.25° increments).23 The authors demonstrated that force transmission supported by the capsular ligament was a median of 1.2% of the applied force with the intact intervertebral disc and increased to 5.1% after nucleotomy over the full extension-flexion cycle. They also found that the capsular ligaments transfer tensile forces mainly in the caudal-posterior direction during extension.23
Facet Biomechanical Functions at Different Spinal Positions
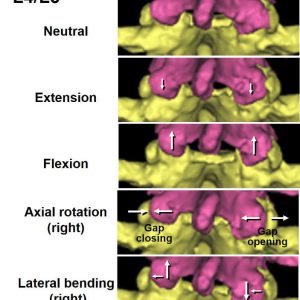
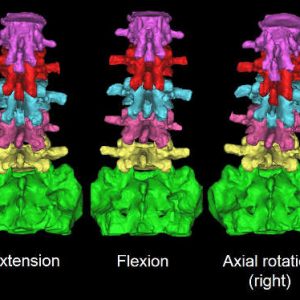
The facet joints fulfill different biomechanical functions in different spinal positions. This section reviews the biomechanical behaviors in flexion-extension, axial rotation and lateral bending (Fig. 10-2).
Flexion-extension
In lumbar extension, the inferior articular processes move inferiorly in reference to the superior articular process of the lower level (Fig. 10-3).24 Kozanek et al. measured the range of motion of the lumbar facet joint (L2/L3 - L4/L5) in vivo in healthy volunteers and reported that the facet joints rotated primarily along the mediolateral axis (average: 2° - 6°) and were translated in the cephalad-caudad direction (average: 2 mm - 4 mm) from full flexion to full extension movements of the trunk with more mobility in the cranial levels.25 Prasad et al. applied +Gz (caudocephalad) impact acceleration to embalmed whole human cadavers sitting on the load cell equipped seat pan and measured strain in the pedicle and lamina and load transmitted through vertebral body in order to estimate the load transmission through the facet joint.26 The results of that study demonstrated that both tensile and compressive loads could be transmitted via the facets or the posterior structures of the lumbar vertebrae and hyperextension of the spine transfers more load to the facets.26 The pressure measurement using the pressure sensitive film by Dunlop et al. showed that the contact area of the articular surface of the facet joint moved to the lower margin of the facet in full extension.20 This study estimated that facet joint carried 10 - 40% of the applied compressive force when in 4° of extension with 1 mm disc height loss.20 A finite element analysis by Schendel et al. also showed that the facet contact site on the inferior articular process of L1 moved inferiorly to a position of tip impingement near the lamina as extension moments increased, and large loads were transmitted through the facet joint during extension (205N at a 10N·m moment and 190N axial load).27 A cadaveric study with intact facet joint capsule by Ivicsics et al. showed that the capsular ligaments carry tensile force in the caudal-posterior directions, especially under the reduced intervertebral disc height condition, in addition to the compressive force transmission through the joint surface in the lumbar extended posture.23
Contact between the tips of the inferior articular process and the lamina or the pars interarticularis can occur in lumbar extension, especially under intervertebral disc height loss conditions as described earlier in this review. Impingement of the capsule due to this bony contact has been postulated as a cause of pain associated with lumbar extension by many authors.6,10,11,17,20 Yang and King found that overloading of the facet joint resulted in rearward rotation of the inferior facets, the tip of which pivoted about the lamina below in compressive testing of the isolated posterior element of the lumbar spine.21 The results of finite element analysis simulations also postulated that hyperextension activities would cause impingement of the inferior process.27,28
In lumbar forward flexion, the inferior articular processes move superiorly about the superior articular process of the lower level. The contact areas are located on the upper tip in the superior articular surface and on the upper and central regions in the inferior articular surface in large flexion.28 The facet joints play an important role in maintaining lumbar stability in forward flexion. During forward flexion, the inferior articular process glides upward and forward upon the superior articular process of the inferior vertebra and the articular surfaces separate at the lower margins of the joint.7 Ivicsics et al. calculated facet joint force vectors in the sagittal plane under a 700N compressive load applied to the motion segment with intact intervertebral disc.23 Ianuzzi et al. measured lumbar facet joint capsule strains during physiological motions using cadaveric spines and reported that the mean principal strains of the joint capsules increased monotonically from full extension to full flexion of the lumbar spine.29 Claeson and Barocas elucidated the existence of in-plane and through-plane shear deformations of the capsular ligament during flexion by finite element models of the lumbar facet joint.30 In this study, the magnitudes of stress and strain were largest across the ligaments between the attachments to the articular facets (i.e., over the joint space). The authors noted that the largest tensile strains were a function of unconstrained motion (i.e., the motion in the anterior direction) and the largest tensile stresses were a function of fiber direction of the capsular ligament.30 Beyond the limit of normal physiologic flexion, the inferior articular process is forced over the superior process, and the compressive loads carried by the facets increase again from those carried in the neutral position.28
Axial rotation
In the lumbar axial rotation, the articular surfaces of the facet joint compress together on one side and tend to open on the other side (Fig. 10-3). For example, with right axial rotation, the left inferior articular process impacts the left superior articular process of the lower vertebra and joint space width in the right facet joint increases. The impaction of the facet joint surfaces limits the range of axial movement and protects excessive torsion of the intervertebral discs. The facets were found to carry large loads during axial rotation and cause the resultant contact force to lie at the posterior edge of the articulating surface at L1/L2.27
Cramer et al. measured facet joint gap distance in the axially rotated position (side-posture positioning) using MRI and demonstrated axial rotation increases the gap distance in the rotated side of the axial rotation (i.e., in the right facet during right axial rotation).31 This study also demonstrated that side-posture manipulation further increases the gap distance.31 An in vivo study on changes in the facet joint space width due to the passive axial rotation has also demonstrated an increased gap distance in the rotated side and a decrease in joint space distance in the opposite side.32 In the gap opening side, the capsular ligament becomes tensed.13 A study using photo-elastic experiments suggested that the superior articular processes are under bending stress during axial rotation by compression in lateral direction and tension in the medial direction.4 The author of this study emphasized a mechanical role of a capsular ligament in the opening side of the facet joint to carry the tensile load during axial rotation.4
Lateral bending
In lateral bending, the inferior articular process glides superior direction in reference to the superior articular process of the inferior vertebra on the convex side of the spinal curve and opposite direction on the concave side (Fig. 10-3).24 Schendel et al. found that the facet joints carry large loads during lateral bending and noted that lateral bending motion was coupled with axial rotation (i.e., left lateral bending was associated with axial rotation which loads the right facet) and the facet resultant contact force location in left lateral bending was in the same area as that for right axial torsion.27 Since coupled motions between lateral bending and axial rotation in the lumbar spine have been reported (for example, left lateral bending to be coupled with right axial rotation),27,33,34 the authors suggested that the axial rotation component associated with lateral bending could be partially responsible for facet loading.27 The coupled motions were also measured in flexion-extension and axial rotation in the aforementioned in vivo study.25
DYSFUNCTION OF THE FACET JOINT
Failure of the facet joint biomechanical function leads to osteoarthritic changes in the fact joint itself and is implicated in disorders of the spinal motion segments. As described in the previous sections, an important function of the facet joint is a restriction of excessive movement of the motion segment in various directions. Failure of this function causes instability and abnormal movement of the motion segment. Similar to other synovial joints, instability can be a major cause of the facet joint osteoarthritis. Regional variation of degenerative changes in the facet joint surface can be explained by changes in contact area/pressure associated with different positions and a decrease in disc height.35,36 Since physiological spinal motions are regulated by coordinated movements of two facet joints and an intervertebral disc in a motion segment, failure of biomechanical function of one component affects functions of other components and the entire motion segment.37
Facetectomy models have been used to demonstrate spinal instability associated with failure of the role of the facet joint to restrict excessive movement and degeneration of the intervertebral disc as the result of instability. Abumi et al. conducted graded facetectomies consisted of unilateral and bilateral medial facetectomies and unilateral and bilateral total facetectomies using fresh human lumbar spinal units.38 In this study, a slight increase in ROM after unilateral medial facetectomy in flexion and an increase in ROM after left unilateral total facetectomy in right axial rotation were found, while no effects were noted in extension and lateral bending even by total bilateral facetectomies. The authors concluded that medial facetectomy does not affect lumbar spinal stability, and conversely, total facetectomy even created unilaterally, makes the lumbar spine unstable.38 A rat model was developed to investigate effects of total facetectomy on intervertebral disc degeneration.39 Degenerative changes of the intervertebral disc were confirmed seven weeks after facetectomy by histology of the disc and increased the roughness of the endplate measured by three-dimensional (3D) micro-CT. This study demonstrated that the total facetectomy mechanically induces intervertebral disc degeneration without direct injury to the disc.39
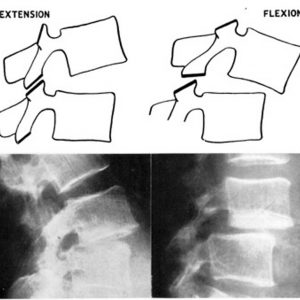
Although the etiology of degenerative spondylolisthesis of the lumbar spine is not well understood, there is a consensus that failure of the facet joint function to limit anterior translation of the motion segment is one of the important factors to cause this spinal disorder. The term “spondylolisthesis” dates back to the 1850s as coined by Kilian and the modern concept of “degenerative spondylolisthesis” was described as a disorder which causes translation in the anterior direction of the cranial vertebra with respect to the caudal one with the neural arch intact by Newman in 1955.40,41 The mechanism of the anterior slippage has been already proposed in the first English paper on degenerative spondylolisthesis by Macnab in 1950.42 The author described that the facet joints hook round anteriorly - like the letter J - and form bars resisting forward displacement, although they may be sagittal posteriorly. He hypothesized that the anterior slippage is unusual because the posterior joints seldom lie in a TRUE sagittal plane. Therefore, he proposed an “over-riding” mechanism of the facet joint during flexion to explain the anterior slippage (Fig. 10-4). In this mechanism, the facet joints lying in an oblique or the coronal plane act as bony bars preventing dislocation, which can occur only by over-riding or fracture of the facets.42 However, Macnab could not prove this concept by a radiographic study. After a CT scan has been developed, the sagittal orientation can be measured in the transverse plane. Although the previous studies using CT images demonstrated more sagittally oriented facet joint in degenerative spondylolisthesis patients, the reported facet angles of the sagittal orientation do not indicate TRUE sagittal orientation. Without the TRUE sagittal orientation in the facet joint, anterior translation in the transverse plane should be blocked with a taper-lock mechanism. Therefore, movement of the inferior facet in the out-of-transverse plane is expected to cause anterior slippage in degenerative spondylolisthesis as Macnab postulated.
Newman and Stone proposed a concept that “slipping will only occur, the neural arch being intact, when the facets give way.”40 The finding that surgical resection of the facet joints can result in spondylolisthesis indicates that altered morphology of the facet joint may have a factor in degenerative spondylolisthesis.38,43,44 Following the development of CT, assessment of facet orientation in transverse planes was of interest among investigators on degenerative spondylolisthesis. Grobler et al. first assessed the role of the lumbar facet morphology as a possible etiology of degenerative spondylolisthesis using CT images.45 The authors measured facet morphology of normal subjects at five different levels and proposed the measurement at the level of the superior endplate of the caudad vertebra is the most representative of the overall morphology of the facet joint. After that, many investigators used this slice level for the measurement of the facet joint. Grobler et al. also set a parameter of “shape dimension,” which corresponds to “bony bar” or “hook” described by Macnab in 1950. However, this parameter has received little attention in the studies on degenerative spondylolisthesis. In fact, Love et al. stated that a major flaw of their own and all previous studies had addressed only one plane of angulation in the measurement of the facet joint, ignoring the inclination of the frontal plane.46 They postulated that the combination of sagittal and horizontal inclination of the facet joint might be significant in the development of degenerative spondylolisthesis.46 In 2009, Toyone et al. evaluated facet orientation differences between cephalad and caudad portions in degenerative spondylolisthesis patients.47 This study measured facet orientation using two transverse CT images taken at cephalad and caudad portions of the facet joint and compared with age-matched non-degenerative spondylolisthesis patients. Although the measurements in this study are not true 3D measurement, this study is the first to evaluate the spatial difference in facet orientation. Like most of the other previous studies, however, this study only measured sagittal orientation using two extreme points at anterior-medial and postero-lateral edges of the facet joint and did not measure shape and size of the facet. This finding may represent a deficiency of anterior-medial cephalad portion of the superior facet in degenerative spondylolisthesis patients.
SUMMARY
The lumbar facet joint exhibits complex 3D geometry including small components within this small joint, which closely link to biomechanical functions of the facet joint and the motion segment in different spinal positions. Over-simplification of the facet joint as a flat joint may prevent proper understanding of the facet joint functions. Despite keen observations of 3D facet joint geometry and consideration of facet joint functions in 3D space reported many decades, even a century ago, development of CT scanning rather tends to limit investigators’ thought in a transverse plane. Axial load transmission and forward translation in motion segments cannot be fully understood without consideration of the special relationships among posterior elements of the lumbar spine. Current imaging modalities allow 3D modeling and re-slicing of the model in the clinical setting; however, valid information cannot be extracted without a proper understanding of the 3D geometry and function of the facet joint.
REFERENCES
- Pal GP, Routal RV. Transmission of weight through the lower thoracic and lumbar regions of the vertebral column in man. J Anat. 1987;152:93-105.
- Gallois J, Japoit T. Architecture interieure des vertebres. Revue de Chirurgie 1925;63:688-708.
- Steindler A. Kinesiology of the Human Body under Normal and Pathological Conditions (1st Ed). Spingfield, IL:Charles C Thomas; 1955.
- Putz R. The functional morphology of the superior articular processes of the lumbar vertebrae. J Anat. 1985;143:181-187.
- Drews S, Matsuura M, Putz R. The trabecular architecture of the superior articular process of the lumbar spine (L2-S1). Surg Radiol Anat. 2008;30(3):209-213.
- Adams MA, Hutton WC. The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. J Bone Joint Surg Br. 1980;62(3):358-362.
- Hadley LA. Anatomico-roentgenographic studies of the posterior spinal articulations. Am J Roentgenol Radium Ther Nucl Med. 1961;86:270-276.
- Beresford ZM, Kendall RW, Willick SE. Lumbar facet syndromes. Curr Sports Med Rep. 2010;9(1):50-56.
- Horwitz T, Smith RM. An anatomical, pathological and roentgenological study of the intervertebral joints of the lumbar spine and of the sacroiliac joints. Am J Roentgenol. 1940;43:173-86.
- Yamashita T, Minaki Y, Ozaktay AC, Cavanaugh JM, King AI. A morphological study of the fibrous capsule of the human lumbar facet joint. Spine (Phila Pa 1976). 1996;21(5):538-543.
- Gorniak G, Conrad W. Lower lumbar facet joint complex anatomy. Austin J Anat. 2015;2:1-8.
- Xu GL, Haughton VM, Carrera GF. Lumbar facet joint capsule: appearance at MR imaging and CT. Radiology 1990;177(22):415-420.
- Boszczyk BM, Boszczyk AA, Putz R, Buttner A, Benjamin M, Milz S. An immunohistochemical study of the dorsal capsule of the lumbar and thoracic facet joints. Spine (Phila Pa 1976). 2001;26(15):E338-343.
- Sato S, Oguma H, Murakami G, Noriyasu S. Morphometrical study of the joint surface and capsule of the lumbar zygapophysial joint with special reference to their laterality. Okajimas Folia Anat Jpn. 2002;79(1):43-53.
- Gellhorn AC, Katz JN, Suri P. Osteoarthritis of the spine: the facet joints. Nat Rev Rheumatol. 2013;9(4):216-224.
- Twomey L, Taylor J. Age changes in lumbar intervertebral discs. Acta Orthop Scand. 1985;56(6):496-499.
- Adams MA, Hutton WC. The mechanical function of the lumbar apophyseal joints. Spine (Phila Pa 1976). 1983;8(3):327-330.
- Ahmed AM, Duncan NA, Burke DL. The effect of facet geometry on the axial torque-rotation response of lumbar motion segments. Spine (Phila Pa 1976). 1990;15(5):391-401.
- Pal GP, Routal RV. A study of weight transmission through the cervical and upper thoracic regions of the vertebral column in man. J Anat. 1986;148:245-261.
- Dunlop RB, Adams MA, Hutton WC. Disc space narrowing and the lumbar facet joints. J Bone Joint Surg Br. 1984;66(5):706-710.
- Yang KH, King AI. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine (Phila Pa 1976). 1984;9(6):557-565.
- el-Bohy AA, Yang KH, King AI. Experimental verification of facet load transmission by direct measurement of facet lamina contact pressure. J Biomech. 1989;22(8-9):931-941.
- Ivicsics MF, Bishop NE, Puschel K, Morlock MM, Huber G. Increase in facet joint loading after nucleotomy in the human lumbar spine. J Biomech. 2014;47(7):1712-1717.
- Jegapragasan M, Cook DJ, Gladowski DA, Kanter AS, Cheng BC. Characterization of articulation of the lumbar facets in the human cadaveric spine using a facet-based coordinate system. Spine J. 2011;11(4):340-346.
- Kozanek M, Wang S, Passias PG, et al. Range of motion and orientation of the lumbar facet joints in vivo. Spine (Phila Pa 1976). 2009;34(19):E689-96.
- Prasad P, King A, Ewing C. The role of articular facets during +Gz acceleration. J Appl Mech 1974;41(2):321-326.
- Schendel MJ, Wood KB, Buttermann GR, et al. Experimental measurement of ligament force, facet force, and segment motion in the human lumbar spine. J Biomech. 1993;26(4-5):427-438.
- Shirazi-Adl A, Drouin G. Load-bearing role of facets in a lumbar segment under sagittal plane loadings. J Biomech. 1987;20(6):601-613.
- Ianuzzi A, Little JS, Chiu JB, Baitner A, Kawchuk G, Khalsa PS. Human lumbar facet joint capsule strains: I. During physiological motions. Spine J. 2004;4(2):141-152.
- Claeson AA, Barocas VH. Computer simulation of lumbar flexion shows shear of the facet capsular ligament. Spine J. 2017;17:109-119.
- Cramer GD, Gregerson DM, Knudsen JT, Hubbard BB, Ustas LM, Cantu JA. The effects of side-posture positioning and spinal adjusting on the lumbar Z joints: a randomized controlled trial with sixty-four subjects. Spine (Phila Pa 1976). 2002;27(22):2459-2466.
- Yang KH, An HS, Ochia RS, et al. In vivo measurement of changes in lumbar facet joint width during torsion. Washington, DC: 51st Annual Meeting of the Orthopaedic Research Society; 2005.
- Gregersen GG, Lucas DB. An in vivo study of the axial rotation of the human thoracolumbar spine. J Bone Joint Surg Am. 1967;49(2):247-262.
- White AA, Panjabi MM. Clinical Biomechanics of the Spineed. Philadelphia, PA: J.B. Lippincott Company; 1978.
- Tischer T, Aktas T, Milz S, Putz RV. Detailed pathological changes of human lumbar facet joints L1-L5 in elderly individuals. Eur Spine J. 2006;15(3):308-315.
- Simon P, Espinoza Orías AA, Andersson GB, An HS, Inoue N. In vivo topographic analysis of lumbar facet joint space width distribution in healthy and symptomatic subjects. Spine (Phila Pa 1976). 2012;37(12):1058-1064.
- Fujiwara A, Tamai K, Yamato M, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
- Abumi K, Panjabi MM, Kramer KM, Duranceau J, Oxland T, Crisco JJ. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976). 1990;15(11):1142-1147.
- Fukui D, Kawakami M, Cheng K, et al. Three-dimensional micro-computed tomography analysis for spinal instability after lumbar facetectomy in the rat. Eur Spine J. 2017;26(8):2014-2020.
- Newman PH, Stone KH. The etiology of spondylolisthesis. J Bone Joint Surg Br. 1963;45:39-59.
- Newman PH. Spondylolisthesis, its cause and effect. Ann R Coll Surg Eng. 1955;16(5):305-323.
- Macnab I. Spondylolisthesis with an intact neural arch; the so-called pseudo-spondylolisthesis. J Bone Joint Surg Br. 1950;32-B(3):325-333.
- Shenkin HA, Hash CJ. Spondylolisthesis after multiple bilateral laminectomies and facetectomies for lumbar spondylosis. Follow-up review. J Neurosurg. 1979;50(1):45-7.
- Sienkiewicz PJ, Flatley TJ. Postoperative spondylolisthesis. Clin Orthop Rel Res. 1987;(221):172-180.
- Grobler LJ, Robertson PA, Novotny JE, Pope MH. Etiology of spondylolisthesis. Assessment of the role played by lumbar facet joint morphology. Spine (Phila Pa 1976). 1993;18(1):80-91.
- Love TW, Fagan AB, Fraser RD. Degenerative spondylolisthesis. Developmental or acquired? J Bone Joint Surg Br. 1999;81(4):670-674.
- Toyone T, Ozawa T, Kamikawa K, et al. Facet joint orientation difference between cephalad and caudad portions: a possible cause of degenerative spondylolisthesis. Spine (Phila Pa 1976). 2009;34(21):2259-2262.