Hardeep Singh and Isaac L. Moss
INTRODUCTION
The axial spine is separated into three regions: cervical, thoracic, and lumbar which are comprised of 7, 12, and 5 vertebrae, respectively.1 The intervertebral disc (IVD) separates each vertebra except C1-C2. Three articulations are present at each level consisting of two facet joints formed from the articulation of the superior and inferior articular processes as well as the IVD at each level. These articulations collectively allow polyaxial motion during physiological loading of the spinal column. The facets joints are true synovial joints similar to other articulations with the ability to resist shear forces during loading. The IVDs are a major component of the spinal column, accounting for one-third of its height.2,3
IVD Structure
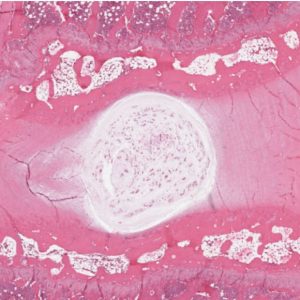
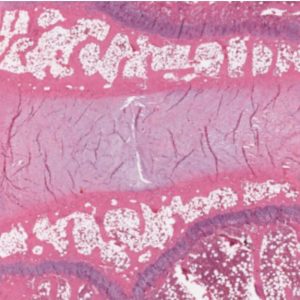
IVDs are fibrocartilaginous pads that primarily serve a mechanical role transmitting physiologic load as well as providing flexibility to the spine, thus allowing flexion, extension, and lateral bending.2-5 There are three distinct regions that comprise the IVD: the vertebral endplates confining the IVD superiorly and inferiorly, the annulus fibrosis (AF), and the nucleus pulposus (NP)4,6 (Figs. 2-1A and B). The annulus forms the thick outer ring of the IVD which encompasses and surrounds the inner gelatinous core known as the nucleus pulposus.3,7
The annulus is formed of 15-25 concentric lamellae with collagen fibers running parallel within each lamella and oriented approximately 60° to the vertical axis.2,3,8,9 The orientation of the collagen fibers alternates in adjacent lamellae, and the lamellae are bound and held together via elastic fibers.2,8,9 Elastic fibers are present throughout the disc, and their orientation varies depending on the region of the disc. Ultimately, they contribute to the mechanical functioning of the IVD.8 The variable orientation and organization of the elastic fibers within the IVD correlate heavily with regards to the differential loading patterns in the IVD.8 With physiological flexion and extension, the IVD height will vary up to 60% and its recovery to a natural state is mediated in part by the elastic fibers.10 The outer layers of the annulus contain elongated thin fibroblastic-like cells which are aligned parallel to the collagen fibers. These cells are more oval like in the inner layers of the annulus and contain cytoplasmic extensions which are thought to function as mechanoreceptors.3
The inner gelatinous core, nucleus pulposus, contains primarily type II collagen fibrils oriented in a random fashion, interspersed within a proteoglycan-rich interfibrillar matrix.11 The proteoglycan-rich matrix allows the IVD to dissipate compressive forces. Chondrocyte-like cells are interspersed throughout the nucleus pulposus, however, at a low density.
IVD Development
Embryologically, the spinal column is formed from a concentration of mesenchymal cells in somites and the central notochord. The segmentation of the central notochord gives rise to condensed and non-condensed regions. The condensed regions form the vertebral bodies, whereas the non-condensed regions form the IVDs. At each disc level, the condensed region is replaced by the mesenchymal cells to form vertebral bodies and cartilaginous end plates.4 The notochordal cells within the noncondensed regions proliferate and synthesize an extracellular matrix rich in glycosaminoglycans forming the embryonic nucleus pulposus. This proteoglycan-rich matrix generates an osmotic gradient, entrapping water within the IVD. The surrounding mesenchyme gives rise to the organized lamellae which comprise the annulus rich in type I collagen.4
The density of the notochordal cells within the nucleus declines as the IVD ages and is replaced by rounded cells resembling articular cartilage chondrocytes.6 At the time of birth, the density of notochordal cells is approximately 2,000 cells/mL. However, it begins to decline significantly following birth. The notochordal cells undergo apoptosis via a Fas-mediated mitochondrial caspase-9 activation, leaving approximately 100 cells/m3 at one year of age and virtually none by late childhood.12 The decline in notochordal cell density leads to migration of chondrocyte cells from cartilaginous endplates in an attempt to populate the nucleus pulposus. These alterations in the composition result in a more firm and rigid nucleus secondary to collagen fibril accumulation.4,6 The chondrocyte cells produce proteoglycans and type II collagen in an attempt to maintain homeostasis.
Blood vessels that enter the IVDs superiorly and inferiorly to provide nutrition, however, start to recede with age causing the IVD to rely primarily on diffusion through the end plates and AF for nutrition. The IVD becomes a relatively acellular, avascular, and stiffer structure with no capacity for regeneration or repair, leading to IVD degeneration with aging.
Biology of IVD
The behavior of individual components in the spinal column is dictated by its biochemical composition. The IVD contains numerous types of collagens (I, II, III, V, VI, IX, XI, XII and XIV), with concentrations varying with the region, age, and severity of degeneration.4,13 Approximately 60% of the dry weight of the AF is comprised of collagen fibers, while 20% of the dry weight of the NP is comprised of collagen fibers.4 Type I and II collagen fibers provide the bulk of the tissue structure while type V, VI, IX, XI, XII, and XIV contribute to the matrix.4 The outermost layers of the annulus fibrosis are comprised mainly of type I and II fibrillar frameworks, with a high density of type I fibers. Collectively type I and type II collagen fibers account for approximately 80% of total collagen in the IVD. The density of type I collagen fibers decrease with progression towards the NP while the density of type II collagen fibers increases.
The extracellular matrix of the IVD contains a wide variety of proteoglycans including aggrecan, versican, hyaluronan, decorin, biglycan, fibromodulin, lumican, and perlecan.4 They account for approximately 50% of the dry weight of the NP and 20% of the dry weight of the AF.4 Aggrecan is the most abundant proteoglycan in the IVD allowing it to resist compressive loads. Chondroitin sulfate, a glycosaminoglycan, is the most abundant side chain linked to a core protein on aggrecan molecules at birth. However, the concentration of chondroitin sulfate decreases while keratin sulfate increases with aging. The reciprocal production of keratin sulfate and chondroitin sulfate as the IVD ages is thought to be secondary to the lack of oxygen supply to the centrally growing disc as a result of both growth and decline in vascularity. Chondroitin sulfate requires glucuronic acid for its formation which in turn requires oxygen for oxidation, while production of keratin sulfate does not require oxygen.14 The rise in keratin sulfate further contributes to the osmotic gradient of the IVD.
Cells in the IVDs produce aggrecan which interacts with hyaluronan forming proteoglycan aggregates. As the IVD ages, the proteoglycan aggregates undergo degradation and the ratio of proteoglycan aggregates to total proteoglycan declines.4,15,16 The mature IVD contains a large quantity of non-aggregating proteoglycans which subsequently undergo degeneration.4 The subsequent accumulation of proteoglycan aggregates in the IVD results in cross-linking and accretion of advanced glycation end products, which are not easily scavenged from the disc.17 The degradation of the proteoglycan aggregates and generation of a large quantity of non-aggregating proteoglycans is thought to be a precursor to disc degeneration.15,16
The IVD contains matrix proteins that assist in cell-matrix interactions and cell function. Specifically, these matrix proteins include fibronectin and elastin.8,18 Fibronectin functions specifically in the organization of the extracellular matrix and cell-to-cell interactions via integrins.18 With the aging of the IVD, there is molecular degradation and accumulation within the disc. In degenerated discs, the levels of fibronectin are elevated and are present as proteolytic fragments.18 Accumulation of these end products shifts the balance towards catabolism via stimulation of metalloproteases and cytokines, hence becoming one of the driving forces in disc degeneration.
Homeostasis is maintained by the balance in the proteolytic enzymes and their inhibitors. Proteolytic enzymes produced by the IVD are responsible for the removal of damaged molecules from the disc. Primarily these proteolytic enzymes belong to the ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs), matrix metalloproteinases (MMPs), and aggrecanases family.19 Inhibitors of proteolytic enzymes called tissue inhibitors of metalloproteinases (TIMPs) work in concert with proteolytic enzymes to maintain homeostasis through tissue turnover. Age-related changes in the IVD lead to an imbalance in the production of proteolytic enzymes and their inhibitors, resulting in a primarily catabolic metabolism. Changes in the biochemical composition of the IVD result in impaired swelling within the disc, consequently, affecting the exchange of nutrients and waste products. Because the cells in the IVD are diffusion dependent for nutrition, they start undergoing apoptosis, and production of type I collagen is increased, changing the microenvironment. These cascading events lead to disc degeneration and formation of a less compliant disc.20
Also, with age, the nutrition supply to the vertebral endplate is diminished secondary to a compromise in the vascular supply. In the healthy young IVD, nutrition is supplied to the cartilaginous endplates and disc via blood vessels. However, as the IVD ages, vascularity is compromised as a result of the calcification in the vertebral endplates.4 With vascular compromise, nutrition supply is decreased causing a conversion to anaerobic metabolism. Production of lactic acid via anaerobic metabolism results in an alteration of the normal pH within the IVD and the generation of an acidic environment, favoring an environment for proteinases over cellular proliferation and matrix production.21
Biomechanics
Biomechanics of the spinal unit are dictated by its structure which consists of two facets joints, supra- and interspinous ligaments, the IVD, and the superior and inferior vertebral endplates. The physiological load is transmitted during axial compression through the vertebral endplates to the IVD, uniformly dispersing stress. The biochemical composition of the IVD allows it to endure the physiological loads. In the physiologically intact IVD, the AF and the vertebral endplates encompass the NP allowing for uniform stress distribution within the well hydrated NP.
In the supine unloaded state, the NP attracts and holds fluid secondary to its hydrostatic pressure and osmotic gradient provided by the microenvironment. With physiological loading of the spine, force is transmitted through the endplates to the IVD leading to a rise in intradiscal pressure. The force is uniformly transmitted to the AF where hoop stresses cause changes in the collagen network organization and deformation occurs resulting in a decrease in the disc height.
As demonstrated in work by Nachemson and Morris, intradiscal pressure is highest in the unsupported sitting position and lowest in the supine position.22 With the removal of the load, the hydrostatic and osmotic pressures within the IVD allow for an influx of fluid, thereby returning the disc to its unloaded state.23 However, with continual loading, viscoelastic changes occur with outflux of fluid from the disc, leading to decreased disc height over time. The IVD has a regular diurnal rhythm with loss of height during the day and with physiological loading and recovery at night. Disruption of this diurnal rhythm results in the development of degenerative disc disease, hence a decrease in intradiscal pressure.22 The biomechanical loading of the IVD influences the cellular viability and matrix, and deviation leads to a cycle of altered cellular function and morphology.21,24,25 Physiological loading of the IVD leads to stimulation of proteoglycan and TIMPs production. Whereas with pathological loading, there is decreased production of proteoglycan and increased production of proteolytic enzymes.21,26 The biomechanical balance of loading and unloading the spinal column contributes significantly to the IVD metabolism, and alterations in this balance lead to unfavorable changes in the cellular matrix, resulting in disc degeneration.4,27,28 Clinically, this translates to deterioration in the quality of life of the patient presenting with pain.
Discogenic low back pain, originating from the IVD, is a well-described source of pain and major disability. However, the anatomic and structural etiology of discogenic pain is poorly understood. Fagan et al. investigated the innervation of various regions of the lumbar disc and proposed that innervation was concentrated in the peri-annular connective tissue and central endplate.29 The majority of the nociceptive fibers in the IVD are concentrated in the outer rim of the annulus fibrosis and posterior longitudinal ligament (PLL). Anatomical studies of spine innervation demonstrate that various sensory pathways exist with pain transmission through nociceptive fibers arising from the sinuvertebral nerve.30 Nociceptive fibers transmit pain segmentally by communication through the sympathetic system via the dorsal root ganglia and by non-segmental route through the paravertebral sympathetic chain.30
Etiology of Intervertebral Disc Degeneration
Disc degeneration is known to impact a patient's quality of life significantly, yet there is no universally accepted definition. A single etiology is not solely responsible for disc degeneration; instead, it is thought to be secondary to exposure to various exogenous and endogenous factors. These can be divided into mechanical factors, genetic predisposition, and nutritional effects.31
Abnormal mechanical loading was thought to be the initiating factor in the onset of disc degeneration. However, various studies have failed to clearly demonstrate a direct relationship between disc degeneration and mechanical factors including heavy labor, physical work, and lifting.31-34 Various animal and clinical studies have not been able to conclusively demonstrate a direct correlation, suggesting that the etiology of disc degeneration is complex and multifactorial.31,35
Several studies have investigated the genetic predisposition to disc degeneration and suggested that heritability accounts for approximately 52-74% for disc disease.36-38 The genetic makeup of an individual plays a key role in the maintenance of disc integrity. Specific polymorphisms and mutations in genes can result in heightened degeneration, even with the application of otherwise harmless forces.31,36
Polymorphisms in the genes encoding for aggrecan, type I, II, and IX collagen genes have been known to lead to a higher risk for multilevel degeneration.39,40 Genetic mutations in the interleukin-6 (IL-6) have been identified and associated with discogenic pain.41 Genetic encoding mutations in the cytokine interleukin-1 (IL-1) have been recently found to be associated with a higher risk of low back pain, disk herniation, and degeneration of the disc.42,43 IL-1 is an inflammatory cytokine which is associated inflammatory reactions and increased expression of MMP-1 and MMP-3.31 MMP-3 functions in the degradation of the matrix, and polymorphisms in the genes encoding for MMP-3 have been identified. These are associated with matrix degradation and acceleration of degenerative changes in the discs. Takahashi et al. investigated the human MMP-3 promoter 5A/6A polymorphism and identified the 5A allele as being a possible risk factor associated with the acceleration of degenerative disc disease in the lumbar disc.44
Nutritional effects are another vital factor which contributes to the process of disc degeneration. With aging, nutritional supply to the IVD becomes limited secondary to the avascularity which correspondingly impacts the removal of waste products. Accumulation of waste products, specifically lactate, and decreased oxygen concentrations result in alternations in the microenvironment of the IVD hindering the anabolic process of proteoglycan synthesis.45,46 These alternations result in increased cell death and failure to maintain the microenvironment in the IVD, ultimately resulting in disc degeneration.47
Biology of Disc Degeneration
Numerous groups have attempted to define disc degeneration as a process of premature aging resulting in pain and deterioration of the quality of life.6 Numerous cellular changes occur with aging involving a change in the cell type, cell density, increased cell death, cell senescence, and change in cellular phenotype.28 The outer annulus fibrosis contains elongated fibroblast-like cells whereas the inner layers of annulus and nucleus fibrosis contain spheroidal and chondrocyte-like cells. The annulus fibrosis cell type remains constant whereas significant changes occur in the nucleus pulposus. The notochordal cellular density in the nucleus pulposus declines steadily from birth to an almost undetectable level by childhood.28,48,49 Chondrocyte cell density starts to rise in the nucleus pulposus as cells start to migrate from the cartilaginous endplates and inner annulus fibrosis.50 The decline in the notochordal cell density negatively impacts the maintenance of a healthy nucleus pulposus. Nomura et al. demonstrated the potential of notochordal cells in preventing IVD degeneration in a white rabbit model. They injected intact nucleus pulposus or nucleus pulposus cells into the IVD and demonstrated that injection of nucleus pulposus and nucleus pulposus cells retards IVD degeneration.51 As the density of notochordal cells declines, so does the fluid-like matrix produced by them, leaving behind a solid cartilaginous matrix produced by chondrocytic cells.4,52 With a decrease in the compliance of the IVD, the stress distribution is disturbed, leading to eccentric loading and progressive damage to the IVD. Load bearing is shifted to the posterior elements, resulting in facet hypertrophy and osteoarthritic changes secondary to the increased load borne by the facet joints.
Annular cells change from fibrocytic spindle-shaped to a rounded and chondrocyte-like with a stellate appearance and multiple branching processes.53,54 As the cellular phenotype changes with aging and degeneration, so do its function. These cells become less responsive and sensitive as compared to cells from non-degenerative discs. As demonstrated by Kang et al., the cells from degenerative discs are less sensitive to interleukin-1β administration; generating lesser amounts of matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2.55 Their response to the mechanical load is altered secondary to the changes in the cellular morphology.
Cells in the nucleus pulposus rely on diffusion of nutrients from the cartilaginous endplates. Aging and vessels’ calcification lead to a decline in the number of normal cells dividing and increase in the apoptosis of cells in the nucleus pulposus.56-58 Cellular senescence ensues as normal cells stop dividing, resulting in decreased ability to maintain the IVD matrix, causing disc aging and degeneration.27,59 Cellular senescence can be either replicative senescence or stress-induced premature senescence. Replicative senescence is secondary to telomere shortening as the cell undergoes repeated divisions; whereas, stress-induced premature senescence occurs due to exposure to pathological loads, reactive oxygen-species, or cytotoxic cytokines.28 The role of cellular senescence in disc aging and degeneration has been demonstrated by numerous studies. Le Maitre et al. demonstrated increased expression of senescence associated β-galactosidase, increased expression of P16INK4A (increased in cellular senescence), and a decline in replicative potential in human disc cells.27
Treatment Strategies
A number of treatment strategies exist to address disc degeneration, ranging from biological therapies to nonoperative and operative management. Nonoperative management is the mainstay treatment option for IVD degeneration as a significant number of patients improve with nonoperative management. Operative management that involves decompression, fusion, or arthroplasty is reserved for a limited number of patients who have failed conservative treatment options and remain limited in their daily living activities secondary to the pain.
Biological therapy involves the delivery of biologically active molecules to the degenerating IVD in the hopes of regeneration or at least cessation of degeneration. Biological therapy can involve local delivery of bioactive molecules, gene therapy to alter gene expression, implantation of autologous cells for regeneration, and delivery of mesenchymal stem cells.31 Delivery of growth factors, gene therapy, stem cells, and bioactive materials for tissue engineering may support and provide an environment conducive to IVD regeneration in otherwise degenerating IVD.60
Injection of bioactive molecules and factors, such as growth factors and proteins, directly into the IVD aims to regenerate the IVD or serves to prevent further degeneration.31 Bioactive molecules can be subdivided into anticatabolics, mitogens, morphogens, and intracellular regulators.61 Anticatabolic molecules act to inhibit degradative enzymes within the IVD, effectively decreasing matrix loss.61 Mitogens act as growth factors to increase cell proliferation and thus matrix production.62 Chondrogenic morphogens belong to the TGF-beta and BMP growth factor family. They act as anabolic factors to upregulate cell metabolism and in turn upregulate the synthesis of collagen, Sox9, aggrecan, and glycosaminoglycans.61,63 Intracellular regulators act within a cell to increase secretion of cytokines, which subsequently act to upregulate matrix production.61
Thompson et al. demonstrated an in vivo increase in proteoglycan synthesis by NP cells following exogenous administration of growth factors TGFβ1.63 An et al. investigated the in vivo effects of intradiscal administration of osteogenic protein-1 (OP-1) and transforming growth factor-beta (TGF-β) into the IVDs of rabbits. Intradiscal administration stimulated an increase in the IVD heights and increased proteoglycan content in the IVD at 2, 4, and 8 weeks.64 Kawakami et al. evaluated the intradiscal administration of OP-1 in a rat disc degeneration model and found the greater production of extracellular matrix and inhibition in the pain-related behavior.65 Intradiscal administration of bioactive molecules and factors appears to be an appealing treatment strategy to address disc degeneration. However, it is limited by the quantity of molecules administered. For a sustained effect and response, a sustained administration of the bioactive molecules and a sufficient quantity of healthy cells in the IVD is required for a response to be produced.31 Healthy cells are capable of generating a response to the bioactive molecules and produce appropriate extracellular matrix proteins, including collagen and proteoglycans. In a severely degenerated disc, administration of bioactive molecules may have a limited response secondary to a low healthy cell density.
Gene therapy is another modality available which aims to genetically alter the IVD to enhance production of various matrix molecules. It serves as another valuable treatment strategy to address IVD degeneration. Gene therapy can be performed directly via in vivo delivery of a gene into the IVD with the prospect that the cells in the IVD will uptake the gene and enhance gene expression. Gene therapy can also be performed indirectly with ex vivo gene therapy in which the desired cell is removed and the gene is incorporated ex vivo and reimplanted into the IVD.66 Genes can be delivered into the IVD via viral or non-viral vectors to enhance delivery and increase gene uptake.31 Viral vectors have the capability to integrate the desired gene into the cell’s genome and enhance gene expression. However, use of viral vectors has raised concerns related to immune response and potential for spread of disease. Non-viral vectors are protein complexes which have lower gene expression and limited effectiveness as compared to viral vectors because they are not incorporated into the cell’s genome and are susceptible to degradation.
Nishida et al. studied the feasibility of gene therapy by using human TGFβ1 to transduce healthy rabbit IVDs via an adenoviral vector. Immunohistochemical studies for human TGFβ1 demonstrated extensive and intensive staining for TGFβ1 and a 30-fold increase in active TGFβ1 production and a 100% increase in proteoglycan synthesis in cells isolated at 1 week.67 Various factors including members of the BMP family growth factors and Sox family have been used to induce cell differentiation to the appropriate lineage. Leckie et al. investigated the injection of adeno-associated virus serotype 2 (AAV2) vector carrying genes of bone morphogenetic protein 2 (BMP2) or tissue inhibitor of metalloproteinsase 1 (TIMP1) into the nucleus pulposus of an in vivo rabbit model to study the course of IVD degeneration. MRI and histologic evidence demonstrated that the treatment groups with AAV2-BMP2 or AAV2-TIMP1 delayed the degenerative changes and may serve as an alternative to IVD regeneration.60 Paul et al. studied the delivery of an adenoviral vector expressing Sox9 in cultured human degenerated IVD cells as well as IVD of three rabbits. The adenoviral vector successfully transduced the cells and led to an increase in Sox9 and type 2 collagen production.68
Implantation of autologous cells remains another viable biological treatment option, with the primary aim to complement or replenish the decreasing cell population secondary to aging and degeneration.69 This process requires the ex vivo expansion of autologous cells and implantation into the degenerated disc. The difficulty arises with obtaining the autologous cells as the degenerating disc does not have sufficient quantity of healthy cells for expansion and harvesting from another IVD would result in damage and subsequent degeneration.31 Herniated nucleus pulposus tissue is a potential cell source that could be harvested without the fear of causing further damage. Expansion and cultivation of the disc cells in a three-dimensional matrix has been demonstrated to maintain the cell morphology and enhance matrix production as compared to cells cultured in a monolayer scaffold.70,71
Tissue engineering using mesenchymal stem cells is another biological treatment option which avoids various difficulties encountered during injection of growth factors, molecules, and gene therapy. Mesenchymal stem cells can be isolated from bone marrow, fat, and muscle.72 Use of mesenchymal stem cells is attractive as they can be manipulated into various lineages (muscle, bone, fat, cartilage, tendon, ligaments) thus being ideal candidates for use in the treatment of disc degeneration.31
Henrikson et al. investigated the survival and function of human mesenchymal stem cells transplanted into injured IVDs in a porcine model. The transplanted human mesenchymal stem cells survived for at least six months and demonstrated expression of chondrocyte markers and mRNA expression of collagen IIA, IIB, versican, aggrecan, and Sox9 indicating differentiation into disc-like cells.73 Orozco et al. conducted a pilot study to examine the efficacy of treatment with mesenchymal stem cells in ten patients with chronic back pain. Autologous expanded bone marrow mesenchymal stem cells were injected into the NP, and the patients were clinically followed for one year. A remarkable improvement in pain and disability was noted; the water content in the IVD remained elevated at the one year mark as seen on MRI. However, the disc height was not recovered.74
Tissue engineering using biomaterials is another modality to address IVD regeneration which incorporates the use of IVD regenerative therapies with the support of scaffolds or matrices.60 The scaffold provides a 3-dimensional structure which serves to essentially mimic the extracellular matrix environment promoting cellular adhesion, migration, proliferation, and differentiation.75 Depending on its purpose, the scaffold can be designed to carry bioactive molecules, drugs, or even progenitor cells in a controlled and sustained delivery system.75 A feasible scaffold for tissue engineering should be easy to fabricate, have the ability to control its mechanical and degradation properties, and have the ability to incorporate bioactive molecules or drugs.60
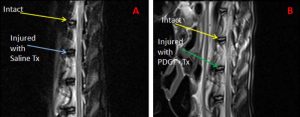
Risbud et al. immobilized mesenchymal stem cells in a 3-dimensional alginate gel and showed that the maturing mesenchymal stem cells formed into a spherical shape and demonstrated nucleus-pulposus like transcripts including high expression of collagen II, XI, aggrecan, decorin, and biglycan.76 Paglia et al. investigated the in vivo effects of platelet-derived growth factor BB (PDGF-BB) in a thiol-modified hyaluronic acid (THMA) hydrogel based model of IVD degeneration. They induced IVD degeneration in a rabbit model via puncturing and four weeks following injury the IVD was treated with either PDGF-BB or PDGF-BB delivered in a THMA hydrogel. A significant decreased in disc degeneration was demonstrated with PDGF-BB treatment and found that when delivered in a TMHA hydrogel scaffold, apoptosis was prevented, and the disc structure and biomechanical function of the disc were maintained77 (Fig. 2-2).
There are currently clinical trials using a variety of cellular and molecular therapies to reverse symptomatic intervertebral disc degeneration underway. The results of these studies have yet to be presented; however, a new treatment option may soon be available to patients with this common and debilitating condition.
CONCLUSION
The IVD is a major component of the spinal column, whose function is dictated by the complex interaction between its biochemical composition and biomechanics of the spinal unit. The IVD forms from a concentration of mesenchymal stem cells and central notochord. It’s intricate biology and chemical composition allow it to resist physiological forces. Aging results in various changes, including a decline in notochordal cells, the decline in vascularity, a decrease in proteoglycan synthesis and accumulation of degradation end products all of which cumulatively disrupt the homeostasis within the IVD. With the disruption of IVD homeostasis, normal physiologically innocuous stimuli result in further disc damage leading to the process of IVD degeneration which clinically translates into discogenic pain. Treatment of IVD degeneration is initially nonoperative with operative management reserved for a limited number of patients. Biological therapy options are attractive because they act to prevent further disc degeneration and possibly stimulate disc regeneration.
REFERENCES
- Miller M, Thompson S. Miller's Review of Orthopaedics. Philadelphia, PA: Elsevier Health Sciences; 2015.
- Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003; 5(3):1.
- Raj PP. Intervertebral disc: anatomy‐physiology‐pathophysiology‐treatment. Pain Pract. 2008; 8(1):18-44.
- Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976). 2004; 29(23):2691-2699.
- Nachemson A, Elfstrom G. Intravital dynamic pressure measurements in lumbar discs. A study of common movements, maneuvers and exercises. Scand J Rehabil Med Suppl. 1970; 1:1-40.
- Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976). 2006;31(18):2151-2161.
- Roberts S, Menage J, Urban JP. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine (Phila Pa 1976). 1989;14(2):166-174.
- Yu J, Winlove PC, Roberts S, Urban JP. Elastic fibre organization in the intervertebral discs of the bovine tail. J Anat. 2002;201(6):465-475.
- Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine (Phila Pa 1976). 1990;15(5):402-410.
- Pearcy MJ, Tibrewal SB. Lumbar intervertebral disc and ligament deformations measured in vivo. Clin Orthop Relat Res. 1984;(191):281-286.
- Inoue H. Three-dimensional architecture of lumbar intervertebral discs. Spine (Phila Pa 1976). 1981;6(2):139-146.
- Kim KW, Kim YS, Ha KY, et al. An autocrine or paracrine Fas-mediated counterattack: a potential mechanism for apoptosis of notochordal cells in intact rat nucleus pulposus. Spine (Phila Pa 1976). 2005;30(11):1247-1251.
- Eyre D, Matsui Y, Wu JJ. Collagen polymorphisms of the intervertebral disc. Biochem Soc Trans. 2002;30(6):844-848.
- Taylor JR, Scott JE, Cribb AM, Bosworth TR. Human intervertebral disc acid glycosaminoglycans. J Anat. 1992;180(Pt 1):137-141.
- Jahnke MR, McDevitt CA. Proteoglycans of the human intervertebral disc. Electrophoretic heterogeneity of the aggregating proteoglycans of the nucleus pulposus. Biochem J.1988;251(2):347-356.
- DiFabio J, Pearce RH, Caterson B, Hughes H. The heterogeneity of the non-aggregating proteoglycans of the human intervertebral disc. Biochem J. 1987;244(1):27-33.
- Verzijl N, DeGroot J, Ben ZC, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46(1): 114-123.
- Oegema TR Jr, Johnson SL, Aguiar DJ, Ogilvie JW. Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine (Phila Pa 1976). 2000;25(21): 2742-2747.
- Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35(4):652-655.
- Hutton WC, Elmer WA, Boden SD, et al. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine (Phila Pa 1976). 1999; 24(15):1507.
- Ishihara H, Warensjo K, Roberts S, Urban JP. Proteoglycan synthesis in the intervertebral disk nucleus: the role of extracellular osmolality. Am J Physiol. 1997;272(5):C1499-C1506.
- Nachemson A, Morris JM. In vivo measurements of intradiscal pressure. Discometry, a method for the determination of pressure in the lower lumbar discs. J Bone Joint Surg Am. 1964; 46:1077-1092.
- Broberg KB. Slow deformation of intervertebral discs. J Biomech. 1993;26(4-5):501-512.
- Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine (Phila Pa 1976). 2000;25(12):1477-1483.
- Lotz JC, Hsieh AH, Walsh AL, Palmer EI, Chin JR. Mechanobiology of the intervertebral disc. Biochem Soc Trans. 2002;30(6):853-858.
- Sauerland K, Raiss RX, Steinmeyer J. Proteoglycan metabolism and viability of articular cartilage explants as modulated by the frequency of intermittent loading. Osteoarthritis Cartilage. 2003;11(5):343-350.
- Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007; 9(3):R45.
- Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6(3):247-261.
- Fagan A, Moore R, Vernon Roberts B, Blumbergs P, Fraser R. ISSLS prize winner: the innervation of the intervertebral disc: a quantitative analysis. Spine (Phila Pa 1976). 2003;28(23):2570-2576.
- Edgar MA. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surgery Br. 2007;89(9):1135-1139.
- Paesold G, Nerlich AG, Boos N. Biological treatment strategies for disc degeneration: potentials and shortcomings. Eur Spine J. 2007;16(4):447-468.
- Sandover J. Dynamic loading as a possible source of low-back disorders. Spine (Phila Pa 1976). 1983;8(6):652-658.
- Heliovaara M. Risk factors for low back pain and sciatica. Ann Med. 1989;21(4):257-64.
- Deyo RA, Bass JE. Lifestyle and low-back pain. The influence of smoking and obesity. Spine (Phila Pa 1976). 1989;14(5):501-506.
- Videman T, Battie MC. The influence of occupation on lumbar degeneration. Spine (Phila Pa 1976). 1999;24(11):1164-1168.
- Battie MC, Videman T, Gibbons LE, Fisher LD, Manninen H, Gill K. 1995 Volvo Award in clinical sciences. Determinants of lumbar disc degeneration. A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine (Phila Pa 1976). 1995;20(24):2601-2612.
- MacGregor AJ, Andrew T, Sambrook PN, Spector TD. Structural, psychological, and genetic influences on low back and neck pain: a study of adult female twins. Arthritis Rheum. 2004;51(2):160-167.
- Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42(2):366-372.
- Doege KJ, Coulter SN, Meek LM, Maslen K, Wood JG. A human-specific polymorphism in the coding region of the aggrecan gene. Variable number of tandem repeats produce a range of core protein sizes in the general population. J Biol Chem. 1997;272(21):13974-13979.
- Kawaguchi Y, Osada R, Kanamori M, et al. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine (Phila Pa 1976). 1999;24(23):2456-2460.
- Noponen-Hietala N, Virtanen I, Kartuned R, et al. Genetic variations in IL6 associate with intervertebral disc disease characterized by sciatica. Pain. 2005;114(1-2):186-194.
- Solovieva S, Kouhia S, Leino-Arjas P, et al. Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology. 2004;15(5):626-633.
- Solovieva S, Leino-Arjas P, Saarela J, Luoma K, Raininko R, Riihimaki H. Possible association of interleukin 1 gene locus polymorphisms with low back pain. Pain. 2004;109(1-2):8-19.
- Takahashi M, Haro H, Wakabayashi Y, Kawa-uchi T, Komori H, Shinomiya K. The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br. 2001;83(4):491-495.
- Bartels EM, Fairbank JC, Winlove CP, Urban JP. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine (Phila Pa 1976). 1998;23(1):1-7; discussion 8.
- Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13(8):695-701.
- Horner HA, Urban JP. 2001 Volvo Award Winner in Basic Science Studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine (Phila Pa 1976). 2001;26(23):2543-2549.
- Pazzaglia UE, Salisbury JR, Byers PD. Development and involution of the notochord in the human spine. J R Med. 1989;82(7):413-415.
- Wolfe HJ, Putschar WG, Vickery AL. Role of the notochord in human intervetebral disk. I. fetus and infant. Clin Orthop Relat Res. 1965;39: 205-212.
- Kim KW, Lim TH, Kim JG Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine (Phila Pa 1976). 2003;28(10):982-90.
- Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Relat Res. 2001;(389):94-101.
- Cappello R, Bird JL, Pfeiffer D, Bayliss MT, Dudhia J. Notochordal cell produce and assemble extracellular matrix in a distinct manner, which may be responsible for the maintenance of healthy nucleus pulposus. Spine (Phila Pa 1976). 2006;31(8):873-82; discussion 883.
- Johnson WE, Roberts S. Human intervertebral disc cell morphology and cytoskeletal composition: a preliminary study of regional variations in health and disease. J Anat. 2003;203(6):605-12.
- Tolonen J, Gronblad M, Vanharanta H, et al. Growth factor expression in degenerated intervertebral disc tissue. An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J. 2006;15(5):588-596.
- Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine (Phila Pa 1976). 1997;22(10):1065-1073.
- Bernick S, Cailliet R. Vertebral end-plate changes with aging of human vertebrae. Spine (Phila Pa 1976). 1982;7(2):97-102.
- Holm S, Maroudas A, Urban JP, Selstram G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8(2):101-19.
- Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993:11(5):747-57.
- Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976). 1995;20(11):1307-1314.
- Vadalà G, Russo F, Di Martino A, Denaro V. Intervertebral disc regeneration: from the degenerative cascade to molecular therapy and tissue engineering. J Tissue Eng Regen Med. 2015;9(6):679-690.
- Yoon ST, Patel NM. Molecular therapy of the intervertebral disc. Eur Spine J. 2006;15(Suppl 3):379-388.
- Masuda K, Oegema TR Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine (Phila Pa 1976). 2004;29(23):2757-2769.
- Thompson JP, Oegema TR Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine (Phila Pa 1976). 1991;16(3):253-260.
- An HS, Takegami K, Kamada H. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine (Phila Pa 1976). 2005;30(1):25-31; discussion 31-2.
- Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Chubinskaya S, Yoshida M. Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine (Phila Pa 1976). 2005;30(17):1933-1939.
- Nishida K, Gilbertson LG, Robbins PD, Evans CH, Kang JD. Potential applications of gene therapy to the treatment of intervertebral disc disorders. Clin Orthop Relat Res. 2000;(379 Suppl):S234-41.
- Nishida K, Kang JD, Gilbertson LG, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine (Phila Pa 1976). 1999;24(23):2419-2425.
- Paul R, Haydon RC, Cheng H, et al. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine (Phila Pa 1976). 2003;28(8):755-763.
- Richardson SM, Hoyland JA, Mobasheri R, Csaki C, Shakibaei M, Mobasheri A. Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J Cell Physiol. 2010;222(1):23-32.
- Maldonado BA, Oegema TR Jr. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992;10(5):677-690.
- Sato M, Asazuma T, Ishihara M, et al. An atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS-scaffold) for the culture of annulus fibrosus cells from an intervertebral disc. J Biomed Mater Res A. 2003;64(2):248-256.
- Gafni Y, Turgeman G, Liebergal M, Pelled G, Gazit Z, Gazit D. Stem cells as vehicles for orthopedic gene therapy. Gene Ther. 2004;11(4):417-426.
- Henriksson HB, Svanvik T, Jonsson M. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976). 2009;34(2):141-148.
- Orozco L, Soler R, Morera C, Alberca M, Sanchez A, Garcia-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011; 92(7):822-828.
- Spadaccio C, Rainer A, Trombetta M, et al. Poly-L-lactic acid/hydroxyapatite electrospun nanocomposites induce chondrogenic differentiation of human MSC. Ann Biomed Eng. 2009;37(7):1376-1389.
- Risbud MV, Izzo MW, Adams CS, et al., Abstracts 26-30. Spine Journal Meeting Abstracts, 2003:28.
- Paglia DN, Singh H, Karukonda T, Drissi H, Moss IL. PDGF-BB delays degeneration of the intervertebral discs in a rabbit preclinical model. Spine (Phila Pa 1976). 2016;41(8):E449-E458.