Richard D. Guyer, MD
Texas Back Institute
Rationale
- It is natural for the spine to move
- Lumbar fusion is the standard of care for many degenerative spinal conditions including symptomatic disc degeneration
- Lumbar total disc replacement (LTDR) is designed to remove the degenerated tissue and replace it with a device allowing mobility of the segment
- LTDR may have benefit over fusion in that the motion provided by the implant may reduce the incidence of accelerated degeneration of the segment(s) adjacent to the operated level
- Approximately 30% of lumbar fusion patients develop adjacent segment degeneration 10 years after surgery, while LTDR is about 25-50% of that rate
Goals of LTDR
- Significantly reduce pain
- Allow motion of the operated segment
- Reduce rate of accelerated degeneration at the segments adjacent to the operated segment
Device Designs
- Most common designs are metal endplates with a plastic core (ProDisc-L and Charité) that produces motion by the endplates sliding over the domed core in a ball-and-socket manner
· Some are metal-on-metal (Kineflex L and Maverick) with a ball-and-socket type design
· Newer designs use one or more compressible materials to produce motion without any sliding components
- Devices currently in use are implanted via an anterior approach to the lumbar spine, the same approach as for anterior lumbar interbody fusion
· There are devices designed to be implanted from a lateral approach and some two-piece designs are being introduced for posterior approaches; some of these are currently being studied in FDA investigational device exemption (IDE) studies
- Current designs of LTDRs were first used in Germany in the mid 1980s
- The Charité (FDA approved October 2004) and ProDisc-L (FDA approved August 2006) are indicated for single level disease at L3/4, L4/5 and L5/S1; Kineflex L is awaiting approval in 2012; there are other designs currently being evaluated in FDA trials
.jpg)
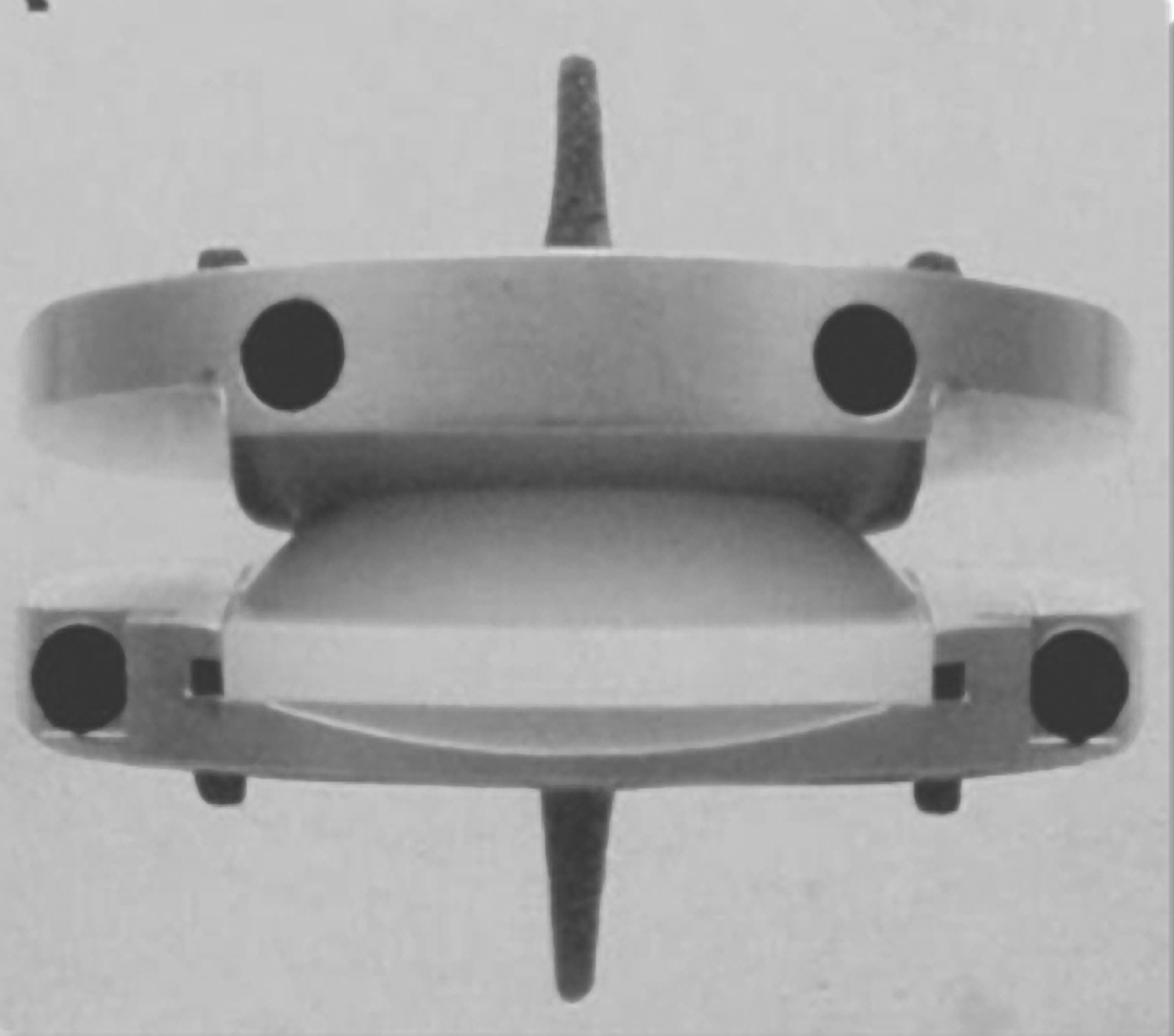
Figure 1. Charité Figure 2. ProDisc-L
Primary Indications for LTDR
- Chronic pain unresponsive to at least 6 months of non-operative care, including physical therapy, medication, activity modification, and injections
- Symptomatic disc degeneration identified by clinical examination and correlating diagnostic studies such as MRI; confirmatory discogram may be helpful, though not mandatory
- Patients with pain following previous laminectomy/discectomy with intact posterior elements
- Patients with significant disc space collapse, provided there is no significant facet disease
Primary Contra-indications for LTDR
- Osteoporosis, fracture, morbid obesity, iatrogenic instability affecting the posterior elements, greater than 3 mm of anterior lithesis, bilateral pars defects, greater than 11° of scoliosis
Clinical Outcome LTDR
- Clinical outcome studies have used various measures, including back pain, leg pain, self-reported function, patient satisfaction, general health status questionnaires, return to work, and medication usage
- Multiple prospective, randomized FDA IDE trials comparing LTDR to fusion with 2- to 5-year follow-up have reported LTDR to produce clinical outcomes non-inferior or superior to fusion1-5
- Charité disc replacement has been discontinued in the U.S. since September 2011
- ProDisc-L is the only implant currently approved and being used in the U.S.
- There have been no studies reporting that LTDR produces results inferior to fusion
· Studies from Europe with 7-year, 10-year, and longer follow-up have reported good outcomes6-8
· Good outcomes were also reported from a national registry that included every LTDR implanted in Switzerland during an approximate 3-year period9
Radiographic Outcome
- LTDR maintains or increases flexion/extension range of motion at the operated segment3,4,10
A.jpg) B
B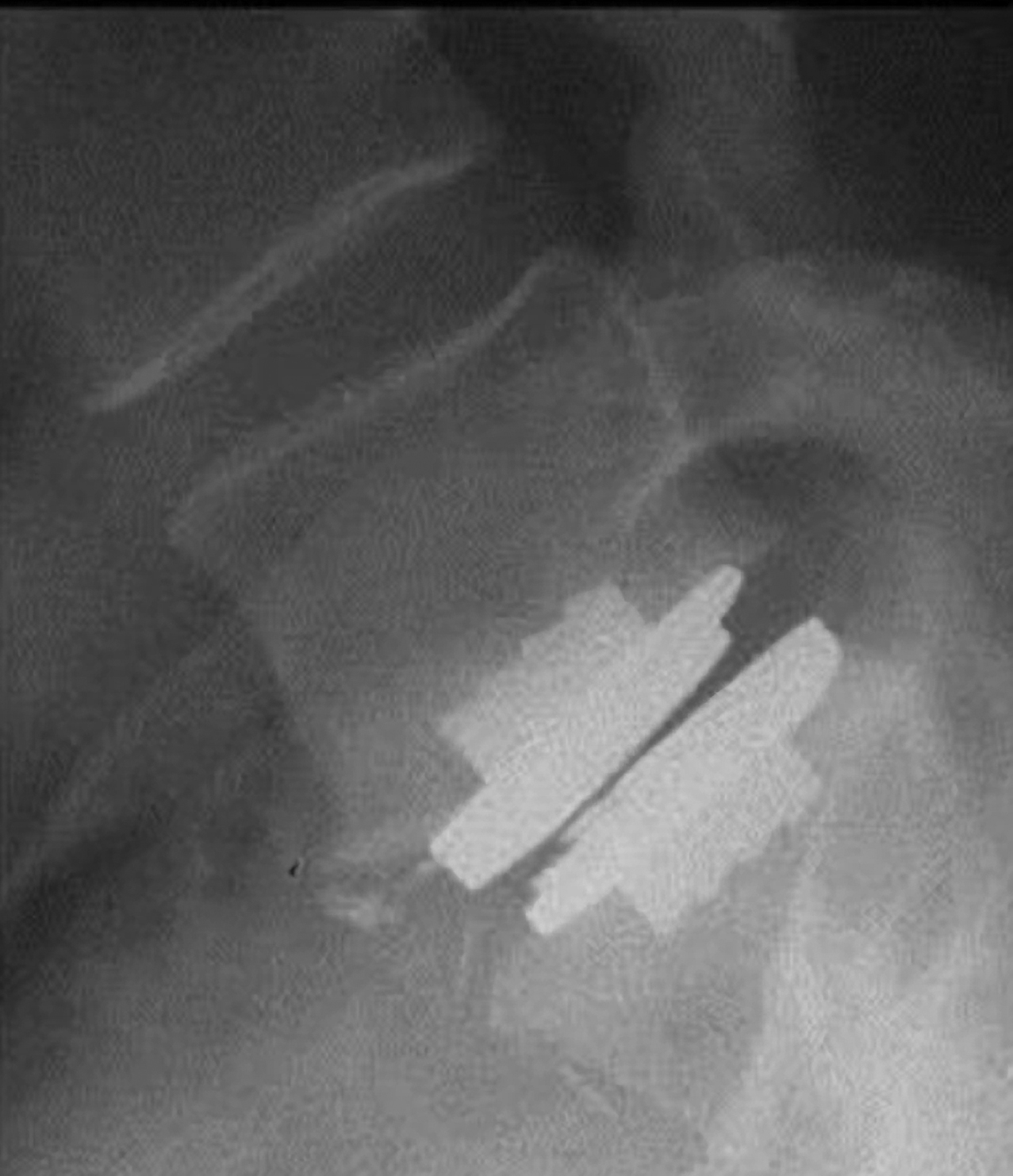 C
C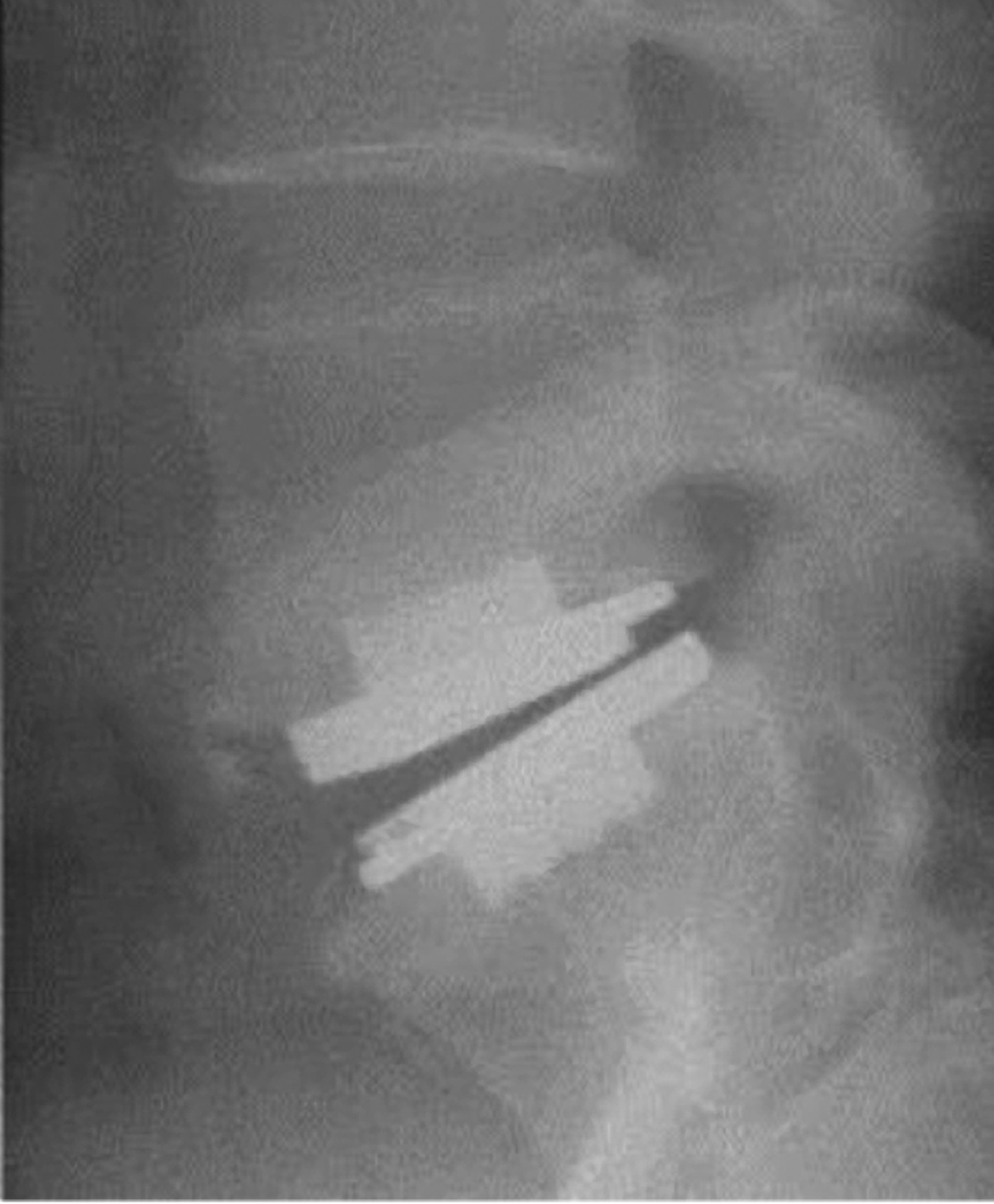
Figure 3. ProDisc-L on AP (A), flexion (B), and extension views (C).
- LTDR is associated with increased height of a collapsed disc space10,11
- Greater range of motion and better clinical outcome are related to more ideal positioning of the implant in the disc space, which generally is centered in the AP view and slightly posterior to the center of the disc in the lateral view10
- Greater motion at the LTDR level has been found to be related to better clinical outcome11
- Greater motion at the LTDR level was associated with reduced adjacent level degeneration12
Complications and Re-operations
- Approach-related complications associated with LTDR are the same as for anterior lumbar interbody fusion and include venous injury; injury to the superior hypogastric plexus, which may result in retrograde ejaculation (usually temporary); ileus; and incisional hernia
- In a meta-analysis of prospective, randomized studies, the complication rate for LTDR was found to be 11.6% vs. 15.0% for fusion;13 the re-operation rates in that meta-analysis were 6.1% for LTDR vs. 8.5% for fusion
- In a series of 1,000 consecutive patients, the re-operation rate was 7.9% (79 patients had 122 procedures);14 only 2.8% of re-operations were at the LTDR level; 48 of the 122 procedures involved the trial, implantation, or revision of a spinal cord stimulator
- There have been reports of LTDR device failure including fragmentation or wear of the Charité polyethylene core;15 the rate of this complication is not clear, as the reports have not included the number of patients undergoing the procedure from which the complications were identified; the original design of the Charité was used only for a brief period before being redesigned; in early European use, the sterilization procedure may have compromised the integrity of the polyethylene core
- Other complications reported include device migration, vertebral body fracture, subsidence, heterotopic ossification, and a delayed hypersensitivity lymphocytic reaction in metal-on-metal implants
Post-operative Course
- LTDR patients can participate in rehabilitation and activities more quickly following surgery than fusion patients (fusion patients need to limit use of their trunk to allow the fusion to incorporate)
- Primary restriction following LTDR is limiting extension for the first 3 months to allow boney ingrowth into the metal endplates, otherwise patients can return to normal activities quickly, except for vigorous sports, which are allowed after 3 months
Cost
- With any new treatment, cost is always a concern; multiple studies have been performed in U.S. comparing the costs of lumbar LTDR vs. fusion16-20
· A variety of methods were used, including economic models, hospital charge data, hospital billing data, hospital payment received, and models based on typical charges for the procedures at a hospital
· Regardless of the patient sample or cost evaluation method used, LTDR was similar or less expensive than fusion, with the only exception being an economic model in which anterior lumbar interbody fusion using autogenous iliac crest (not commonly performed) was found to be less expensive
References
1. Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter food and drug administration investigational device exemptions study of lumbar total disc replacement with the charite artificial disc versus lumbar fusion: Part i: Evaluation of clinical outcomes. Spine 2005;30:1565-75.
2. Guyer RD, McAfee PC, Banco RJ, et al. Prospective, randomized, multicenter food and drug administration investigational device exemption study of lumbar total disc replacement with the charite artificial disc versus lumbar fusion: Five-year follow-up. Spine J 2009;9:374-86.
3. Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter food and drug administration investigational device exemption study of the ProDisc-l total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine 2007;32:1155-62.
4. Gornet MF, Burkus JK, Dryer RF, et al. Lumbar disc arthroplasty with maverick disc versus stand-alone interbody fusion: A prospective, randomized, controlled, multicenter investigational device exemption trial. Spine 2011;36:E1600-E11.
5. Delamarter R, Zigler JE, Balderston RA, et al. Prospective, randomized, multicenter food and drug administration investigational device exemption study of the ProDisc-l total disc replacement compared with circumferential arthrodesis for the treatment of two-level lumbar degenerative disc disease: Results at twenty-four months. J Bone Joint Surg Am 2011;93:1-11.
6. David T. Long-term results of one-level lumbar arthroplasty: Minimum 10-year follow-up of the charite artificial disc in 106 patients. Spine 2007;32:661-6.
7. Lemaire JP, Carrier H, Sariali el H, et al. Clinical and radiological outcomes with the Charite artificial disc: A 10-year minimum follow-up. J Spinal Disord Tech 2005;18:353-9.
8. Tropiano P, Huang RC, Girardi FP, et al. Lumbar total disc replacement. Seven to eleven-year follow-up. J Bone Joint Surg Am 2005;87:490-6.
9. Schluessmann E, Diel P, Aghayev E, et al. Swissspine: A nationwide registry for health technology assessment of lumbar disc prostheses. Eur Spine J 2009;18:851-61.
10. McAfee PC, Cunningham B, Holsapple G, et al. A prospective, randomized, multicenter food and drug administration investigational device exemption study of lumbar total disc replacement with the charite artificial disc versus lumbar fusion: Part ii: Evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine 2005;30:1576-83.
11. Guyer RD, Pettine K, Knight RQ, et al. Radiographic comparison of two lumbar total disc replacement devices: Results from a prospective, randomized, controlled multicenter FDA-regulated trial. Spine Arthroplasty Society. New Orleans, Louisiana: 2010.
12. Huang RC, Girardi FP, Cammisa Jr FP, et al. Long-term flexion-extension range of motion of the prodisc total disc replacement. J Spinal Disord Tech 2003;16:435-40.
13. Yajun W, Yue Z, Xiuxin H, et al. A meta-analysis of artificial total disc replacement versus fusion for lumbar degenerative disc disease. Eur Spine J 2010;19:1250-61.
14. Zigler JE, Guyer RD, Blumenthal SL, et al. Analysis of re-operations after lumbar total disc replacement: Experience in 1,000 consecutive cases beginning with first case experience of 11 surgeons. North American Spine Society. Orlando, Florida, 2010.
15. Kurtz SM, van Ooij A, Ross R, et al. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J 2007;7:12-21.
16. Guyer RD, Tromanhauser SG, Regan JJ. An economic model of one-level lumbar arthroplasty versus fusion. Spine J 2007;7:558-62.
17. Kurtz SM, Lau E, Ianuzzi A, et al. National revision burden for lumbar total disc replacement in the united states: Epidemiologic and economic perspectives. Spine 2010;35:690-6.
18. Levin DA, Bendo JA, Quirno M, et al. Comparative charge analysis of one- and two-level lumbar total disc arthroplasty versus circumferential lumbar fusion. Spine 2007;32:2905-9.
19. Ohnmeiss DD, Hume CS, Blumenthal SL, et al. Cost comparison of total disc replacement vs. Fusion in patients with insurance denial for disc replacement. North American Spine Society. Orlando, Florida, 2010.
20. Patel VV, Estes S, Lindley EM, et al. Lumbar spinal fusion versus anterior lumbar disc replacement: The financial implications. J Spinal Disord Tech 2008;21:473-6.

